Surfactants are reduced. Detergent surfactant. Surfactants. Natural and synthetic. Their advantages and disadvantages
Surface- active substances(Surfactant), possessing wetting, emulsifying and detergent properties, are widely used in industry and, as a consequence, are contained in Wastewater ah the respective industries. [...]
Surfactants (surfactants) or detergents are widely used in industry and in everyday life as detergents. Getting into water bodies with wastewater, they cause foaming, worsen the organoleptic properties of water, disrupt oxygen exchange processes, toxicly affecting the fauna. [...]
Surfactant therapy improves survival and shortens ventilator therapy in infants. While primary surfactant deficiency may be rare in newborn horses, secondary deficiency is likely to be underestimated.
Surfactants are very expensive and this limits their use in veterinary medicine. Hence, there are several published reports on the use of surfactants in equine medicine. Both animal and synthetic surfactants are commercially available. The surfactant dose is divided and each portion is introduced into the endotracheal tube to the patient in various positions. Manual ventilation should be performed immediately after each bolus dose to maximize distribution to the distal airways and alveoli.
SURFACE-ACTIVE SUBSTANCES (SAS) - substances that can accumulate on the interface between phases (media), lowering its free energy ( surface tension). Surfactants are used in industry (for example, in flotation), they are included in detergents (detergents), varnishes and paints, pesticides, food products. Getting into water bodies, surfactants strongly change the properties of the environment and negatively affect life processes. SURFACE WATER - land waters permanently or temporarily located on the earth's surface in a liquid (rivers and temporary streams, lakes, reservoirs, swamps) or solid (glaciers and snow cover) state. [...]
Alternatively, the surfactant can be delivered using a nebulizer, although the effectiveness of this method is unknown. The use of lavage surfactant therapy to remove aspiration debris has been reported. Recent evidence suggests that the addition of polymers such as dextrans to surfactant solutions can also help reduce surfactant inhibition under inflammatory or obstructive conditions.
Surfactant Protective Agents
Surfactants are wetting agents composed of hydrophilic and oleophilic groups that can reduce the surface tension of a liquid and reduce the interfacial tension between two liquids. It dissolves in oil due to CH chains and soluble in water due to polar groups. When these molecules are located at the air-water or oil-water interface, the hydrophilic groups collide with the aqueous phase, while the oleophilic groups indicate the gas and oil phases.
Surfactants and petroleum products are among the most common environmental pollutants. The annual volume of this waste at only 35 machine-building enterprises in Ufa is more than 11,000 m3 per year of spent coolant, 10,000 m3 per year of watered oil products, 600 m3 per year of spent surfactants and washing solutions. [...]
Surfactants can be classified into two main types: ionized and non-ionized. Ionized surfactants can be ionized to ions when dissolved in water. In freeze-drying biological products, surfactants can reduce denaturation during freezing and dehydration caused by stress at the ice-water interface. Surfactants can also act as wet agents during the rehydration process.
V last years surfactants are used in low temperature storage and freeze drying of food, pharmaceutical and biological products. Since non-ionized surfactants have a relatively low critical micelle concentration, they are usually used at a low concentration. Typical surfactants are shown in Table 3. Anionic surfactants, because of their superior foam and foam characteristics, are commonly used as primary surfactants in cleansers.
DETERGENTS (MS) - powders, pastes and liquid solutions used for washing clothes. Modern M.S. consist of several components, including detergent (soap, surfactant), bleach, water glass and washing soda. As part of M.S. an important role was played by phosphates, which, when released into water bodies with domestic wastewater, caused eutrophication of water bodies. At present, recipes have been developed for phosphate-free MS. Modern M.S. can be used in any water, regardless of its hardness, and for washing various fabrics. However, the content of M. with. in domestic wastewater creates many problems during their cleaning and limits the use of sewage sludge (dry residue after cleaning) as fertilizers. At present, work continues on the creation of MS, minimally polluting the environment. [...]
Typical surfactants used in cleansing bars
Since the rods must maintain a "solid" shape, and since the structure must withstand the processing conditions of the bar, the amount of primary surfactants that can be used in bars is very limited. In contrast, surfactants for liquids can have a much wider range of chemicals as they do not have the same limitations as bar. Below is the short review surfactants used in bars and liquid cleansers. Most of the bars made around the world use soap as a cleanser.
Surfactants are compounds that, under certain conditions and at certain concentrations, can alter the degree of water wettability of the surface of various solids. This is the basis for the use of surfactants in many industries and especially in everyday life as detergents and cleaning agents. [...]
In short, oils and fats - each triglyceride, made up of three long-chain fatty components attached to a glycerol molecule, is first converted to fatty acids or fatty acid esters, which then react with alkali to form soap. Common oils used for soap are vegetable oils such as palm oil, palm oil derivatives, rice bran oil, peanut oil, and castor oil in combination with coconut oil or palm kernel oil.
Non-vegetable sources are usually obtained from tall oil. Synthetic surfactants are made from oils, fats or petroleum products, usually through some combination of esterification, ethoxylation and sulfonation, rather than saponification.
Nonionic detergents - derivatives of polyoxyethylenes are one of the active parts of some synthetic detergents. They can be found in industrial wastewater or wastewater from various industries. V surface waters nonionic substances, like anionic detergents, come from waste water. [...]
Examples of synthetic surfactants include sodium cocoyl isethionate, sulfosuccinates, alpha-olefin sulfonates, alkyl glyceryl ether sulfonate, sodium cocoyl monoglyceride sulfate, and betaines. Cleansing sticks with soap are formulated in an alkaline pH range with pH values up to 10-. In contrast, bars with syndite are drafted in a neutral pH range.
Typical surfactants used in cleaning fluids
Liquid detergents often contain a combination of anionic and amphoteric surfactants. Nonionic surfactants and amino acid based surfactants are also found in liquid cleansing systems. Typical anionic surfactants used in cleaning products include soaps and synthetic surfactants such as alkyl ether sulfate, alkyl acyl estionates, alkyl phosphates, alkyl sulfosuccinates, and alkyl sulfonates. Amino acid-based surfactants such as acylglycinates are also increasingly used as primary surfactants in liquid cleaning systems.
Organic substances present in circulating water (in an undissolved and dissolved state), under the same conditions, inhibit the formation of CaCO3 deposits. We can assume that organic matter being in a colloidal state and, as it were, enveloping the nuclei of CaCO3 crystals, to some extent prevent their growth and deposition on the walls of refrigerators; surfactants (detergents) play a similar role. [...]
Commonly used zwitterionic surfactants include cocoamidopropyl betaine and cocoamphoacetate. Alkyl polyglucoside is one of the nonionic surfactants found in some cleaning products. Most liquid detergents are formulated in the neutral to acidic pH range, except those containing soap as the main active ingredient.
Surfactants are chemical substances which reduce the surface tension of the water and thus help to “wet” the surfaces. For this reason, they are commonly referred to as wetting agents. They also facilitate the penetration of the cleaning solution into the sludge.
DETERGENT (S) - surface active synthetic substances used in industry and everyday life as detergents and emulsifiers. They serve as one of the main chemical pollutants of water bodies, since they are hardly decomposed by microorganisms, disrupt the oxygen balance, and have a harmful effect on living organisms. [...]
Surfactant molecules are composed of a hydrophobic tail and a hydrophilic head, as shown in Figure 1. The hydrophobic part of the molecule has an affinity for fats and oils, while the hydrophilic part of the molecule has an affinity for water. These molecules thus emulsify and disperse oils, fats, waxes and pigments. Some of them will precipitate at high pH values and are not suitable for use in combination with strong alkalis.
The polarity of water molecules creates a high surface tension, and as a result, water forms droplets on the surface. The orientation of the surfactant molecules at the interface lowers the surface tension and causes the water droplet to propagate. In a compound detergent, these surfactant effects lead to better penetration of the detergent into the soil sludge.
Synthetic surfactants (SAS) are a group of chemical compounds, the presence of which in wastewater especially threatens the sanitary state of water bodies and sharply negatively affects the operation of treatment facilities. Surfactants appear in wastewater as a result of their widespread use in everyday life and industry as detergents, as well as wetting, emulsifying, leveling, and disinfectants. The greatest application of synthetic surfactants is found in the oil and textile industries. In household detergents, the content of the active agent (surfactant) reaches 20-30%. [...]
This allows the surfactants to solubilize or emulsify fats. On contact with the fatty soil, the hydrophobic ends of the surfactant are incorporated into the fat. As more of these hydrophobic ends penetrate the oily soil, the soil becomes the center of the molecular weight of the micelle, resulting in fat solubilization.
Surfactants are classified into three groups based on the charge of the active part of the molecule. The active part of the anionic surfactant molecule carries a negative charge. This group includes salts of fatty acids and sulfated and sulfonated derivatives of fatty acids. Anionic surfactants work in the same way as soaps, except that they are less susceptible to hard water ions and tend to be foaming.
Synthetic detergents are produced in powder, liquid and paste form. [...]
Synthetic detergents (CMC), the surfactant base of which are usually anionic detergents (alkylbenzenesulfonates, etc.), are widely used in industry and everyday life. To increase the activity of detergents, intracomplex compounds are introduced into their composition, usually sodium tripolyphosphate. Up to 80-85% of surfactants (surfactants) are used for the manufacture of synthetic detergents. The use of these funds inevitably leads to their entry into wastewater. Pollution of water bodies by these runoffs, especially in areas with pronounced turbulence, causes intense foam formation. Cases are known when thick foam covered the surface of the river in a layer of up to 3 m in an area of thousands square meters.[ ...]
The active part of the cationic surfactant molecule carries a positive charge. Quaternary ammonium compounds are included in cationic surfactants. These compounds have limited detergent performance, but have found effective use as disinfectants.
Nonionic surfactants do not ionize and therefore no charged particles are formed. There is a wide range of neionoscopy and therefore these surfactants have a wide spectrum chemical properties... Non-ionoscopy can be low foaming and highly penetrating wetting agents, making them particularly effective in removing oils.
Actually, all surfactants - both used for the production of herbicides and used for the manufacture of detergents as detergents - are surfactants. Surfactants that enhance the effect of herbicides are only those substances that, when added to preparations during their production or to working solutions before use, increase their biological activity. The name surfactants is borrowed from the English-language special literature (surface active agents). In the literature on German these substances are called tensides; both terms are used in Hungary. [...]
Biosurfactants have a wide range of physical and chemical properties, but they have certain properties. All biosurfactants are amphiphilic in nature, containing at least one hydrophilic and one hydrophobic moiety. The hydrophilic moiety can be an ester, hydroxyl, phosphate, carboxyl, or carbohydrate group and is either neutral or negatively charged. There were no cationic biosurfactants, presumably because cationic surfactants are generally quite toxic.
Schwartz, Perry, Birch, Surfactants and Detergents, IL, 1960. [...]
The composition of organic substances in industrial wastewater of modern production is extremely diverse - these are aromatic hydrocarbons, petroleum products, phenols, oils, resins, amino products, pyridine bases, fatty acids, surfactants of synthetic detergents and many others. Despite the fact that the main components of various organic pollutants are the same elements (mainly carbon, hydrogen, oxygen and nitrogen), degree. the harmfulness of one or another organic compound depends on the form of finding, that is, the combination, of these elements in water. Therefore, in order to obtain a complete sanitary and hygienic characteristic of water quality, it is necessary to know the content of certain organic substances in the water. Via modern methods chemical analysis, including spectrographic ones, theoretically it is possible to determine any combination of elements of organic substances in water, but in practice, when analyzing natural and waste waters, this is not done due to the variety of organic compounds in these waters, as well as due to the complexity and cumbersomeness of many analyzes. The content of individual organic substances - pollutants - is established by analysis, as noted above, only in cases where there are assumptions about the presence of these very substances in the water. [...]
The hydrophobic moiety is a fatty acid of 8 to 18 carbon atoms. Due to their amphiphilic nature, surfactants tend to accumulate on surfaces and surfaces. As a result, surfactants reduce the repulsive forces between dissimilar phases on surfaces and surfaces and allow the two phases to mix more easily. In particular, surfactants can reduce surface and interfacial tension. For example, an experienced biosurfactant can lower the surface tension between clean water and air from 73 mNm to less than 30 mNm.
Surfactants mean substances that are part of detergents. This is one or more groups of surface-active agents and several binding components. The former reduce the surface tension of the liquid in which they dissolve and form a stable emulsion or suspension with particles of the removed dirt. Binding components reduce water hardness due to the formation of an alkaline solution with water, in which the detergent properties of surface-active groups are especially effective. [...]
To remove residues of surfactant detergents, glass vessels are rinsed with a mixture of 9 parts of methanol or ethanol and 1 part of concentrated HC1. [...]
Despite the excellent detergency, organic solvents can not always clean the surface of the product from mineral dirt. Therefore, in necessary cases, as well as when fatty layers have poor adhesion to the material of the product, cheaper and safer in operation water-washing solutions and suspensions based on surfactants, soda, soaps, etc. [...]
The function of detergents as surfactants is to remove dirt from the fibers of the material and keep it in the cleaning solution. Since this property is suppressed by calcium and magnesium ions, a "phosphate detergent component" is added to neutralize the action of the latter, which improves the detergent action [...]
Surfactants are widely used in industry and in everyday life as detergents. [...]
The use of synthetic surfactants that replace soap is becoming more widespread in industry and in everyday life. Currently, the most widespread are OP-7, OP-Yu, sulfanol and non-cal, which are oil refined products, and Novost powder (gardinol), which is produced from the fat of the sperm whale. There is still no way to extract these substances from wastewater. [...]
The purpose of surfactants as detergents causes the ingress of almost all of their products into waste water, which, in turn, can pollute surface water bodies, groundwater, soil. Chemical and physicochemical methods of wastewater treatment do not solve the problem of combating water pollution with surfactants, since when using these methods, surfactants, as a rule, are only concentrated or partially destroyed, but do not completely decompose to CO2, H2O and other simple products. The complete destruction of detergents is carried out by microorganisms, on the use of which all biological methods of water purification are based. However, the purification of effluents from surfactants is generally accepted biological methods difficult, since many of these substances are relatively resistant to microbial degradation and pass through sewage treatment plant without changing. At the same time, surfactants, due to their high capacity for foaming, disrupt their work, reducing the rate of sedimentation of activated sludge. The spreading of foam by the wind creates an epidemiological hazard, since pathogenic bacteria, in particular pathogens of intestinal infections, spread along with the foam. The number of bacteria in water bodies during foaming increases greatly due to the fact that extremely favorable trophic conditions are created in the foam. A small amount (0.2-0.4 mg / l surfactant) gives an unpleasant taste and smell drinking water... The formation of foam on the surface of water bodies violates the oxygen regime and causes massive death of the flora and fauna inhabiting them. The study of sanitary and hygienic aspects of water pollution with surfactants is devoted to the monograph by E. A. Mozhaev, which provides data on their effect on water quality, self-cleaning ability of water bodies, human and animal organisms. [...]
TENSIDES (syn. Synthetic surfactants - SPAV, T.) - chemical compounds concentrating on the surface of two media, for example, water and air. T. improve the wettability of surfaces and easily penetrate between dirt particles and the materials on which they are located. T. is the most important ingredient in detergents (washing powders and pastes) and cleaning products. The most famous T. is soap, which has been used by mankind for 5000 years. T. adversely affect the skin, dissolving fats, are slowly destroyed in the environment, and are toxic to many inhabitants of aquatic ecosystems. Currently, T. are being developed, which are rapidly destroyed in water and therefore are less dangerous for environment.[ ...]
Organic solvents are widely used as degreasing and washing solutions: alcohols, gasoline, kerosene and all kinds of compositions based on petrochemical synthesis products. Their use in industrial conditions is associated with a certain degree of explosion and fire hazard; systematic work in the atmosphere, saturated with vapors such substances are harmful to the health of workers. Due to the emergence of a wide range of non-flammable and non-toxic surfactants, the consumption of organic solvents for degreasing has decreased. However, problems have arisen associated with the disposal of waste solutions. Draining them into the sewerage system dramatically increases the degree of wastewater pollution and the cost of their purification. [...]
Additional organic substances entering, for example, open surface water bodies during the processes of technogenesis, cause their eutrophication, i.e. enrichment with biogenic components (Fig. 1.5.4). As a result, water bodies are overgrown with algae, the decomposition of which, after they die off, consumes a large amount of oxygen. The forming sapropel leads to the shallowing of the reservoir, its gradual transformation into a swamp with an active release of biogas. Eutrophication is facilitated by the drift into water bodies of nitrates leached from fertilizers, or phosphates from detergents (detergents). [...]
As emulsifiers, synthetic surfactants are most widely used in the production of synthetic rubber, rubber, plastics, polymers, and synthetic resins. With their help, polymerization of vinyl halides, acetates, ketones, ethers, thioethers and their mixtures is carried out. Alkyl sulfates polyhydric alcohols are also an aid for painting and filling rubber and plastics. Many surfactants are used to stabilize adhesives, suspend solid and water-insoluble insecticides, are used in the leather, pharmaceutical and perfume industries, in addition, they have become an indispensable detergent. [...]
An integral part of synthetic detergents are anionic and nonionic surfactants (surfactants). [...]
SOAP (M.) is the most traditional surfactant, which improves the wettability of the surface with a solution, enhances the electrostatic repulsion between dirt particles and the detergent, and increases the emulsifying and suspending ability of water. [...]
In recent years, synthetic detergents MJI, "Labomid", MC, etc. have become widespread. Preparations ML-51 and ML-52 are a mixture of surfactants with electrolytes: sodium salts of silicon and phosphoric acid... These drugs are available in the form of powder or granules of white and light yellow color. The drug ML-51 is used for jet cleaning of deposits at a solution concentration in water of 10-20 g / l, the drug ML-52 is used for cleaning parts by boiling from resinous deposits at a concentration of 25-35 g / l with a solution temperature of 70-80 ° C. The washing ability of ML preparations is 2-3 times higher than solutions based on caustic soda. [...]
Synthetic detergents, which are based on surfactants and alkaline salts (Labomid 101.203, Temp-1 OOD, etc.), have become widespread during cleaning. When using synthetic detergents, a soda ash aerosol is released as the cleaning solution. Specific emissions of pollutants during washing of parts and assemblies are given in table. 7. [...]
Phosphates are ingredients in many synthetic surfactants (see below) used as detergents and fertilizers. With wastewater, they enter soil, rocks and The groundwater causing various pollution... The harmful effect of phosphates on soils and groundwaters consists in their stimulation of the development of cyanobacteria, "blooming" of water bodies (Chapter 2). [...]
Surfactants that reduce the surface tension of water have a negative effect on living organisms in water. Conventional soaps are anionic surfactants. Surfactants are widely used as stabilizers of dispersed systems (emulsions, suspensions), dispersants, film-forming agents and detergents. [...]
DETERGENTS (from Lat. - I wash) - Chemical compounds that lower the surface tension of water and are used as detergents and emulsifiers. Especially widespread are synthetic surfactants (surfactants), which are part of detergents and cleaning agents (for which a new concept has been introduced abroad - tensides). Diverse application (for washing dishes, fabrics, cars, for personal hygiene) has led to an ever increasing transition to domestic and industrial wastewater. V agriculture Surfactants are used to emulsify pesticides, so they end up in soils and groundwater. [...]
It should be noted that with the appearance of synthetic surfactants in household waters and an increase in the content of phosphorus compounds (as a result of the use of synthetic detergents for washing), they resort to irrigation with purified wastewater of meadow drained areas as a method of post-treatment of household wastewater from these substances ... An example of such solutions are the British sewage treatment plants Norton Green and Langley Mill with a capacity of 2,000 and 1,600 m3 / day, respectively. Actual loads at these stations are 0.16-OD m3 / (m2-day). To ensure the necessary aeration of the soil, irrigation is carried out periodically - with interruptions, no more than 6 times a day. This technique allowed at the Langley Mill station to reduce the concentration of surfactants in biologically treated wastewater from 2.5 to 1 mg / l and thereby increase the overall effect of surfactant removal from 90 to 95.9%. [...]
In a number of cases, attention was drawn to the visible effects caused by detergents, which have recently become widely used in a number of countries for domestic, commercial and industrial purposes. Thus, the total amount of such substances in the main river basins has increased by several milligrams per liter, especially due to droughts. Surfactants have been found in drinking water from such aqueous systems. One of the problems arising from the presence of detergents in the water of major river basins is the problem of foam. In Germany, the Necker River has fallen into disrepute due to the pollution of its water with foam. Due to the extreme variety of detergents available on the market, there is currently insufficient information on their damaging properties; experiments were carried out only to determine the specific radicals of surface-active compounds. [...]
With municipal and partly industrial waters, detergents enter the reservoirs - detergents, synthetic surfactants (SAS). It is high molecular weight organic compounds obtained by sulfonation of various oils, hydrocarbons, high molecular weight alcohols and other substances of petroleum origin. The composition of detergents includes 20-40% surfactants and 60-80% of various additives. [...]
Dirty glass does not wet well plain water, therefore, it is impossible to carry out an effective washing process without the use of special detergents. The detergent formulation includes caustic soda (sodium hydroxide), soda ash (sodium carbonate, sodium carbonate), trisodium phosphate, liquid glass(sodium silicate), sulfates, sodium metasilicate, surfactants and other substances in different combinations and concentrations. So, the concentration of caustic soda can be in the range of 0.65-3%, surfactants 0.2-0.4%, trisodium phosphate 0.3-1.5%, etc. [...]
A very dangerous type of pollution inherent in urban wastewater is detergents, or synthetic surfactants (surfactants), which are strong toxicants that are resistant to biological degradation processes. They are widely used in everyday life and in many industries as detergents, emulsifiers, foaming agents. In economically developed countries, the concentration of surfactants in wastewater reaches 5-15 mg / l. Water is poorly cleaned from detergents and up to 50-60% of their initial amount is discharged into water bodies. In practice, the concentration of these substances in urban wastewater is on average 4-6 mg / l, and in river waters - 0.3-1.0 and even 1.5 mg / l, although the maximum permissible concentration of surfactants in natural waters should not exceed 0.3 mg / l. [...]
In presenting this section, we follow. In addition to oil products, synthetic surfactants are found in coastal waters. In industry and in everyday life, a large number of detergents are used, used as detergents, stabilizers of foams and emulsions, evaporation retardants, etc. Getting into the ocean with wastewater, they also participate in the formation of surface-active films. [...]
The wastewater of textile enterprises, chemical fiber production and a number of others contains impurities of various detergents, dispersants, as well as production waste, which has a significant surface activity, especially in a neutral or slightly alkaline environment. These impurities reduce the surface tension, increase the stability of the foam, which facilitates its removal from the skimmers. Thus, flotation turns out to be an effective integrated method for removing suspensions, emulsions and dissolved surfactants of various structures from wastewater (if the latter effect is the main purpose of wastewater treatment, then in this case it comes not about flotation, but about foam concentration of dissolved substances). It should be borne in mind that flotation treatment of water also causes oxidation of a number toxic substances or blowing them off. Due to this, the general sanitary and hygienic effect of water purification in flotation machines is incomparably higher than the effect of water settling even with the use of coagulants, especially since the introduction of the latter or sorbents directly into the floated water is also often very effective. [...]
The result of measuring the conductivity is also affected by contamination of the electrodes, especially when analyzing wastewater containing surfactants: fats, resins, oils, etc. The measurement results in such cases are doubtful. After the end of the measurement, the contaminated electrodes should be washed first with a solution of a suitable solvent selected in accordance with the type of contamination, then with alcohol or a solution of a synthetic detergent, and finally thoroughly washed with distilled water. The determination of the electrical conductivity of samples, in the analysis of which the influence of interfering conditions may appear, is carried out, as a rule, using electrodes with a shiny surface. [...]
Recently, the problem of foam has emerged in wastewater treatment. This is due to the use of synthetic detergents in industry and in everyday life. The presence of small amounts of surfactants in wastewater causes the formation of stable foam in aeration tanks and a significant decrease in the sedimentation rate of suspended solids. In some cases, a content of 5 mg / l of synthetic detergents in the wastewater is sufficient to create a problem of foaming. [...]
As an example, we present the data on the assessment of the dynamic characteristics of CTS of wastewater treatment in the bleaching shop of the dyeing and finishing production of a textile enterprise from anion-active surfactants, which are widely used in the technology of finishing fabrics as wetting and washing preparations. The change in the concentration of surfactants in the wastewater of such a production depending on the operating time is illustrated in Fig. 3.1. Based correlation analysis changes in the composition of wastewater at the entrance to the cold storage system received autocorrelation function shown in Fig. 3.2. [...]
The disadvantage of soda solutions is a relatively high corrosive ability, which sufficiently limits their use. The appearance in recent years of a wide range of surfactants with excellent detergents has naturally displaced soda solutions from the field of industrial use. The use of detergent compositions based on surfactants in cleaning products, equipment and containers from high-molecular compounds ( epoxy resins, polydieneurethane rubbers, mixtures based on them, etc.). [...]
A water jet is a washing machine into which washing liquid is supplied under a pressure of 0.8-1.2 MPa. The washing liquid washes the inner surface of the tank using the washer's nozzles. A hot (45-70 ° C) aqueous solution of ML-2 detergent is used as a washing liquid, the concentration of which is 0.15-0.35%. The detergent is a composition of synthetic surfactants with electrolyte additives. [...]
Among other methods of combating "harmful tar" in the production of cellulose, it should be noted that it is advisable to clean the cooking acid from cymene, pre-steaming the chips in the boiler in order to remove the turpentine part of the resin, and thoroughly rinsing the cooked cellulose. warm water and separation of liquor therefrom, treatment of cooked cellulose with detergent surfactants with separation of resin by flotation, alkaline extraction of resin from cellulose in multi-stage bleaching followed by washing the cellulose and treating it with nonionic surfactants and polyphosphates. Finally, it should be noted that the fine fraction of fibers (3-4% of the total amount of cellulose), containing the main amount of resin, is separated from cellulose on a gum separator. [...]
Hydromonitors are used for chemical-mechanical cleaning, but solutions of various preparations are used as a working fluid, therefore, the hydrodynamic and thermal effect of a liquid jet on residual contaminants is accompanied by physicochemical processes that facilitate the removal of these contaminants. A variety of substances are used as reagents, the choice of which depends on chemical composition residual dirt and liquid in the container. For example, if the contaminants contain free organic acids, washing is carried out with alkaline solutions, since the interaction of these substances forms soaps that emulsify both inorganic and organic contaminants. In the presence of resinous hydrocarbon deposits in the container, organic solvents are sometimes used, but their use is associated with an increased fire hazard. Detergents containing surfactants, which have a high dispersing ability with respect to dirt of various chemical composition, are widely used. There are a number of complex compositions of detergent solutions, including, along with surfactants, the addition of alkaline solutions or organic solvents. In the case of using surfactants when washing tanks for aviation fuel, they should be completely removed from the tank after washing, since even insignificant additions of these substances to the fuel can significantly impair the efficiency of cleaning it from free water. In fig. 6 shows the scheme of flushing a vertical tank using a set of equipment for mechanical cleaning of tanks (OMZR). [...]
According to the internal structure of particles, micellar colloids are isolated into a separate group, they are also called semi-colloids. They are formed from long-chain organic molecules with diphilic properties: that is, the non-polar radical interacts better with organic (non-polar) liquids, and the polar part of the molecule (carboxyl and other groups) interacts better with polar water molecules. Micelles are formed due to intermolecular dispersion forces, which are manifested during the contact of non-polar parts of molecules. The formation of such colloids is characteristic of aqueous sols of detergents (for example, C17Hs5C001Ma soap) and some organic dyes with large molecules. This group includes synthetic surfactants.
Surfactants (surfactants) are chemical compounds that, concentrating on the interface, cause a decrease in surface tension.
The main quantitative characteristic of a surfactant is surface activity - the ability of a substance to reduce the surface tension at the interface is the derivative of surface tension with respect to the surfactant concentration as C tends to zero. However, the surfactant has a solubility limit (the so-called critical micelle concentration or CMC), upon reaching which, when the surfactant is added to the solution, the concentration at the interface remains constant, but at the same time self-organization of surfactant molecules occurs in the bulk solution (micelle formation or aggregation). As a result of this aggregation, so-called micelles are formed. Hallmark micelle formation is the turbidity of the surfactant solution. Aqueous solutions Surfactants, during micelle formation, also acquire a bluish tint (gelatinous hue) due to the refraction of light by micelles.
Typical surfactants are organic compounds of an amphiphilic structure, i.e., containing atomic groups in a molecule that differ greatly in the intensity of interaction with the environment (in the most practically important case, water). So, surfactant molecules contain one or more hydrocarbon radicals that make up the oleo- or lipophilic part (it is also the hydrophobic part of the molecule), and one or more polar groups - the hydrophilic part. Oleophilic (hydrophobic) groups, weakly interacting with water, determine the tendency of the molecule to transition from an aqueous (polar) medium to a hydrocarbon (non-polar) one. Hydrophilic groups, on the contrary, keep the molecule in a polar medium or, if the surfactant molecule is in a hydrocarbon liquid, determine its tendency to transition to a polar medium. Thus, the surface activity of surfactants dissolved in non-polar liquids is due to hydrophilic groups, and those dissolved in water are due to hydrophobic radicals.
Examples. According to the type of hydrophilic groups, surfactants are divided into ionic, or ionic, and non-ionic, or non-ionic. Ionic surfactants dissociate in water into ions, some of which have adsorption (surface) activity, others (counterions) are adsorption inactive. If anions are adsorptively active, surfactants are called anionic, or anionic, in the opposite case - cationic, or cationic. Anionic surfactants - organic acids and their salts, cationic - bases, usually amines varying degrees substitutions, and their salts. Some surfactants contain both acidic and basic groups. Depending on the conditions, they exhibit the properties of either anionic or cationic surfactants, therefore they are called amphoteric, or ampholytic, surfactants
Ionic surfactants.
Anionic substances make up a large part of the world production of surfactants. This group of tensides is the most incompatible with dirt, and it is this group that is most criticized by soap-phobes. Anionic and cryptanionic compounds (acetyl peptides, lauryl and laureth sulfates of sodium, potassium, magnesium or ammonium) are the best at removing dirt from contact surfaces. Therefore, no effective cleanser can do without them.
People learned to produce this group of detergent substances earlier than others (recall the passage about ashes, which the ancient sages sprinkled on their heads). With the development of progress, anionic tensides began to be cooked from proteins and fats, alkalized (sodium hydrolization) with ash (the most popular alkaline natural raw material is the ash of the Salasola soda tree) and other alkaline compounds. Coconut, palm, rapeseed, soybean oil, pork fat, spermaceti, butter from cow and goat milk are used as raw materials for anionic and cryptanionic surfactants.
The outstanding cleansing qualities of anionic tensides are explained by the structure of their molecules, which consist of two parts - hydrophilic ( loving water) and, conversely, hydrophobic.
The former allow them to dissolve in water (or polar solvents) and be completely washed off by it from the skin surface, while the latter allow them to come into contact with non-polar substances (hydrocarbons, resins, urea, dust, fats, oils). When washing with shampoo or soap, hydrophobic "jaws" bind the trapped dirt particles, placing them in the center of the micelle (a hollow ball formed by a number of molecules, the hydrophilic "tails" of which are directed outward, and inward - hydrophobic "heads").
Fast, complete, high-quality evacuation of dirt from the surface of the skin and its appendages, foaming, bactericidal (elimination of gram-positive microorganisms) and bacteriostatic, lipolytic (dissolution of oxidized fat secretion of the sebaceous glands and resinous-mineral skin contaminants) action.
Industrial surfactants of this type can be divided into a trace. main groups: carboxylic to-you and their salts (soaps), alkyl sulfates (sulfoesters), alkyl sulfonates and alkylarylsulfonates, other products.
In the production of soaps and many ionic and nonionic soap-like surfactants, carboxylic acids, obtained by hydrolysis from vegetable and animal fats, and synthetic fatty acids are used. Resin and fatty to-you of tall oil - a by-product of cellulose production - resin to - you of rosin, among which abietic predominates, are also of industrial importance.
Soaps (sodium, potassium and ammonium) are of the greatest importance as surfactants from salts of monocarboxylic acids fatty to-t RСООН, where R is a saturated or unsaturated normal aliphatic radical with the number of carbon atoms 12-18, and soaps (sodium, less often potassium) resin to-t. Dicarboxylic compounds are also of practical importance, for example. alkenyl succinic, obtained in the industry by condensation of unsaturated hydrocarbons with maleic anhydride.
Alkyl sulfates are usually synthesized by sulfoesterification of higher fatty alcohols or α-olefins, followed by neutralization, respectively, of primary or secondary alkyl sulfuric acids.
Alkylaryl sulfonates, Ch. arr. mono- and dialkylbenzenesulfonates, as well as mono- and dialkylnaphthalenesulfonates make up most of the synthetic. non-active surfactants.
Alkyl sulfonates are usually obtained from saturated C12 - C18 hydrocarbons of normal structure, to-rye sulfochlorinated or sulfo-oxidized, followed by saponification or neutralization of the product.
Cationic IIAB.
Cationic tensides are compounds that dissociate (dissolve) in aqueous solution to form cations (positively charged molecules).
Quaternary ammonium bases are polysaccharides obtained from dairy products, lanolin, potatoes, corn, sugar cane, beets, and sunflowers.
Due to their positive charge, cationic tensides are attracted to negatively charged hair and horny scales of the epidermis, accelerating their wetting, fix on their surface the valuable medicinal components contained in the cosmetic preparation, and also have a bradykinase effect (eliminate irritation, itching, burning, swelling). They capture and hold negatively charged particles and kill gram-negative bacteria.
Cationic tensides are valuable components of cosmetic preparations (shampoos, balms, conditioners): they activate foaming, increase the productivity of oxygen exchange of the skin and hair, emulsify oily and aromatic substances in an aqueous solution, have a bactericidal effect, eliminate the residual electric charge on the hair after washing (antistatic effect ), provide easy combing, styling and increase the efficiency of medicinal components on the skin and hair.
They can be divided into a trace. main groups: amines of various degrees of substitution and quaternary ammonium bases, other nitrogen-containing bases (guanidine, hydrozines, heterocyclic compounds, etc.), quaternary phosphonium and tertiary sulfonium bases.
The raw material for cationic surfactants, which are of economic importance, are amines obtained from fatty to-t and alcohols, alkhalides, as well as alkyl phenols. Quaternary ammonium salts are synthesized from the corresponding long-chain alkyl halides by reaction with tertiary amines, from amines by chloroalkylation, or in other ways from synthetic alcohols, phenols, and phenolic mixtures.
Of great importance as cationic surfactants and as starting products in the synthesis of nonionic surfactants (see below) are not only mono-, but also diamines, polyamines and their derivatives.
Amphoteric surfactants
Manufacturers of quality cosmetics in order to soften the effect of the best, i.e. of anionic cleansing ingredients (in particular, so that anionic surfactants do not damage the fat complex of the hydrolipid mantle) and at the same time do not reduce one iota of the cleansing activity of the drug, they must be added to the soapy formula of co-surfactants. Amphoteric and non-ionic tensides neutralize the acid reaction of anions, promote their rapid decomposition, while increasing the density and reducing the "airiness" (diameter) of the foam bubbles.
Amphoteric surfactants are one of the most expensive ingredients in a lather base. They are obtained by pomace, extraction, infusion, rectification and oxidation of natural raw materials (both vegetable and animal). The most famous raw materials sources of amphoteric tensides, namely cocoamphoacetate, lactate, alpha-amino acids, pectins, waxes, are soapwort, algae, apple pulp, root vegetables (beets, carrots, Jerusalem artichoke), palm oil, dairy products, lanolin.
Even basic knowledge in chemistry gives rise to doubts about the possibility of combining cationic and anionic surfactants in a single formula, since oppositely charged substances, attracting in pairs to each other, reduce their affinity for water (precipitate), and, naturally, the cleansing activity decreases. Only thanks to amphoteric surfactants this problem was resolved: these surfactants, due to their ability to easily give up and attach an electron pair, exhibit both acidic and basic properties, depending on the reaction of the environment in which they are located (in an alkaline environment, they become anions, and in acidic - cations).
properties of amphoteric surfactants
Amphoteric tensides protect the skin and hair from dryness and irritation, restore the stratum corneum and keratin of the hair, soften, increase the elasticity of the connective tissue, make the hair silky, and the lathering agent foam - a creamy texture.
They are obtained from anionic ones by introducing amino groups into them or from cationic ones by introducing acidic groups.
Such compounds, for example RNHCH2CH2COONa, are prepared by reacting a primary amine and methyl acrylate followed by saponification of the ester group with an alkali.
Industrial amphoteric surfactants are produced in small quantities, and their consumption is expanding slowly.
Nonionic surfactants
Compounds that dissolve in water without the formation of ions are called non-ionic. Their group is represented by polyglycolic and polyglycolic esters of fatty alcohols (for example, feistenzide - Disodium Laurethsulfosuccinate - a fluid liquid consisting of citric acid and fatty alcohols). Get nonionic surfactants by oxyethylation vegetable oils(castor, wheat germ, flax, sesame, cocoa, calendula, parsley, rice, St. John's wort). Non-ionic surfactants exist only in liquid or paste form, therefore they cannot be contained in solid detergents (soap, powders).
Aqueous solutions esters fatty acids are a dispersion micelle solution, which is often called "smart soap" because it emulsifies dirt and grease, removing them from the surface of the skin and hair without damaging the protective mantle.
Properties of non-ionic surfactants
This type of surfactant brings softness, safety, environmental friendliness to the detergent (biodegradability of non-ionic tensides is 100%). They stabilize soap suds, have mild thickening properties, have a bradykinase and polishing effect, restoring the outer layers of the epidermis and hair, and contribute to the activation of the action of therapeutic additives of the cleansing agent.
This is the most promising and rapidly developing class of surfactants. At least 80-90% of such surfactants are obtained by adding ethylene oxide to alcohols, alkyl phenols, carboxylic acids, amines, and other compounds with reactive hydrogen atoms. Polyoxyatilene ethers of alkyl phenols are the most numerous and widespread group of nonionic surfactants, including more than a hundred trade names, the most famous are OP-4, OP-7 and OP-10. Typical feedstocks are octyl, ionyl and dodecylphenols; cr. in addition, they use cresols, cresol to-that, β-naphthol, etc. If an individual alkylphenol is taken into the reaction, finished product is a mixture of surfactants with total f-ly RC6H4O (CH2O) mH, where m is the degree of oxyethylation, depending on the molar ratio of the starting components.
All surfactants. can be divided into two categories according to the type of systems they form when interacting with a dissolving medium. Micelle-forming surfactants belong to one category. century, to the other - not forming micelles. In solutions of micelle-forming surfactants c. Above the critical micelle concentration (CMC), colloidal particles (micelles) appear, consisting of tens or hundreds of molecules (ions). Micelles decompose reversibly into individual molecules or ions when the solution is diluted (more precisely, colloidal dispersion) to a concentration below the CMC.
Thus, solutions of micelle-forming surfactants. occupy an intermediate position between true (molecular) and colloidal solutions, therefore they are often called semi-colloidal systems. Micelle-forming surfactants include all detergents, emulsifiers, wetting agents, dispersants, etc.
It is convenient to evaluate the surface activity by the greatest decrease in surface tension divided by the corresponding concentration - CMC in the case of micelle-forming surfactants. Surface activity is inversely proportional to CMC:
The formation of micelles occurs in a narrow range of concentrations, which becomes narrower and more definite as the hydrophobic radicals lengthen.
The simplest micelles of typical semi-colloidal surfactants, for example. fatty salts to - t, at concentrations not too exceeding the CMC, have a spheroidal shape.
With an increase in the surfactant concentration of anisometric micelles, it is accompanied by a sharp increase in the structural viscosity, which in some cases leads to gelling, i.e. complete loss of fluidity.
The action of detergents. Soap has been known for thousands of years, but only relatively recently did chemists understand why it has detergent properties... The mechanism for removing dirt is essentially the same for soaps and synthetic detergents. Let's consider it using the example of table salt, ordinary soap and sodium alkylbenzenesulfonate, one of the first synthetic detergents.
When dissolved in water, table salt dissociates into positively charged sodium ions and negatively charged chloride ions. Soap, i.e. sodium stearate (I), similar substances, as well as sodium (II) alkylbenzenesulfonate behave in a similar way: they form positively charged sodium ions, but their negative ions, in contrast to the chloride ion, consist of about fifty atoms.
Soap (I) can be represented by the formula Na + and C17H35COO–, where 17 carbon atoms with hydrogen atoms attached to them are stretched into a winding chain. Sodium alkylbenzenesulfonate (Na + C12H25C6H4SO3–) has about the same number of carbon and hydrogen atoms. However, they are located not in the form of a winding chain, as in soap, but in the form of a branched structure. The significance of this distinction will become clear later. It is important for the detergent action that the hydrocarbon part of the negative ion is insoluble in water. However, it is soluble in fats and oils, and it is thanks to fat that dirt sticks to things; and if the surface is completely free of grease, dirt does not linger on it.
Negative ions (anions) of soap and alkylbenzenesulfonate tend to concentrate at the water / fat interface. The water-soluble negatively charged end remains in the water, while the hydrocarbon portion is immersed in the fat. For the interface to be as large as possible, the fat must be present in the form of tiny droplets. As a result, an emulsion is formed - a suspension of droplets of fat (oil) in water (III).
If there is a film of fat on a hard surface, then on contact with water containing a detergent, the fat leaves the surface and passes into the water in the form of tiny droplets. Soap and alkylbenzenesulfonate anions are found at one end in water and at the other end in fat. The dirt retained by the fat film is removed by rinsing. So in a simplified form, you can imagine the action of detergents.
Any substance that tends to collect at the oil-water interface is called a surfactant. All surfactants are emulsifiers because they promote the formation of an oil-in-water emulsion, i.e. "Mixing" oil and water; they all have detergent properties and form foam - after all, foam is like an emulsion of air bubbles in water. But not all of these properties are expressed in the same way. There are surfactants that foam abundantly but are mild detergents; there are also those that hardly foam, but are excellent detergents. Synthetic detergents are synthetic surfactants with extra high cleaning power. In the industry, the term "synthetic detergent" generally means a composition comprising a surfactant, bleaches and other additives.
Soaps, alkylbenzenesulfonates and many other detergents, where the anion dissolves in fats, are called anionic. There are also surfactants in which the cation is fat-soluble. They are called cationic. Typical cationic detergent, alkyldimethylbenzylammonium (IV) chloride

is a quaternary ammonium salt containing nitrogen bound to four groups. The chloride anion always remains in water, therefore it is called hydrophilic; hydrocarbon groups associated with positively charged nitrogen are lipophilic. One of these groups, C14H29, is similar to the long hydrocarbon chain in soap and alkylbenzenesulfonate, but attached to a positive ion. Such substances are called "reverse soaps". Some of the cationic detergents are highly antimicrobial; they are used in detergents intended not only for washing, but also for disinfection. However, if they cause eye irritation, then when they are used in aerosol formulations, this circumstance should be reflected in the instructions on the label.
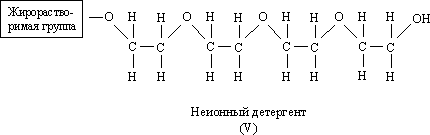
Another type of detergent is non-ionic detergents. The fat-soluble group in the detergent (V) is something like the fat-soluble groups in alkylbenzenesulfonates and soaps, and the remainder is a long chain containing many oxygen atoms and an OH group at the end, which are hydrophilic. Generally, non-ionic synthetic detergents show high detergency but weak foam.
Surfactants (Synthetic Surfactants) are a large group of compounds that differ in their structure and belong to different classes. These substances are able to adsorb at the interface and consequently lower the surface energy (surface tension). Depending on the properties exhibited by surfactants when dissolved in water, they are divided into anionic substances (the active part is the anion), cationic (the active part of the molecules is a cation), ampholytic and nonionic, which do not ionize at all.
It is no secret that the main active components of washing powders are surfactants. In truth, these active chemical compounds, entering the body, destroy living cells by disrupting the most important biochemical processes.
Synthetics are the future? Apparently yes. In confirmation of this, surfactants are being improved more and more, there are so-called nonionic surfactants, the biodegradability of which reaches 100%. They are more effective at low temperatures, which is important for gentle washing modes. Since many man-made fibers cannot withstand high temperatures. In addition, wash more cold water saves energy, which is more relevant every day. Unfortunately, most non-ionic surfactants are liquid or pasty and are therefore used in liquid and pasty detergents. Nonionic surfactants are introduced into powdered SMS in the form of additives 2-6% wt. An important advantage of synthetic surfactants is that they do not form calcium and magnesium salts that are poorly soluble in water. This means that they wash equally well in both soft and hard waters. The concentration of synthetic detergents, even in soft water, can be much lower than soaps made from natural fats.
Probably from products household chemicals we are most familiar with synthetic detergents. In 1970, for the first time worldwide, more synthetic detergents (CMCs) were produced than conventional natural soaps. Every year its production decreases, while the production of SMS is constantly increasing.
For example, in our country, the dynamics of growth in the production of SMS can be displayed by the following data: in 1965 they produced 106 thousand tons, in 1970 - 470 thousand tons, and in 1975 almost one million tons will be produced.
Why is the production of natural, sound, soap, which has served people with faith and truth for many years, so declining? It turns out he has a lot of flaws.
Firstly, soap, being a salt of a weak organic acid (more precisely, a salt formed by a mixture of three acids - palmitic, margaric and stearic) and a strong base - caustic soda, hydrolyzes in water: sya (that is, it breaks down) into an acid and alkali. Acid reacts with hardness salts and forms new, already water-insoluble salts, which fall out in the form of a sticky white mass on clothes, hair, etc. This not very pleasant phenomenon is well known to everyone who tried to wash or wash in hard water ...
Another product of hydrolysis, alkali, destroys the skin (defatting it, leading to dryness and the formation of painful cracks) and reduces the strength of the fibers that make up various tissues. Polyamide fibers (nylon, nylon, perlon) are destroyed by soap especially intensively.
Secondly, soap is a relatively expensive product, since its production requires food raw materials - vegetable or animal fats.
There are other, less significant disadvantages of this substance, which, until recently, was completely irreplaceable in everyday life.
In contrast to natural soaps, synthetic detergents have undeniable advantages: greater detergency, hygiene and economy.
On the international market, there are now about 500 names of synthetic detergents produced in the form of powders, granules, flakes, pastes, and liquids.
The production of SMS gives a great national economic effect. Experiments have shown that one ton of synthetic detergents replaces 1.8 tons of 40 ° / o-th laundry soap made from valuable food raw materials. It is estimated that one ton of SMS saves for Food Industry 750 kg of vegetable fat.
The use of SMS in household allows you to reduce labor costs for manual and machine washing by 15-20%. At the same time, the strength and original consumer properties of the fabric (whiteness, color brightness, elasticity) are preserved much better than when using ordinary laundry soap.
It must be said that SMS is not only intended for washing clothes. There are special products for washing and cleaning various household items, synthetic toilet soaps, hair shampoos, foam detergents for baths, into which biostimulants are introduced that have a tonic effect on the body.
The main component of all these products is a synthetic surfactant (surfactant), the role of which is the same as that of organic salt in ordinary soap.
However, chemists have long known that an individual substance, no matter how universal it may be, cannot satisfy all the requirements imposed on it. Small additions of other, accompanying substances help to detect very useful qualities... That is why all modern SMS are not individual surfactants, but compositions that may include bleaches, fragrances, foam regulators, biologically active substances and other components.
The second most important component of modern synthetic detergents are condensed, or polymer, phosphates (polyphosphates). These substances have a number of useful properties: they form water-soluble complexes with the metal ions present in water, thereby preventing the appearance of insoluble mineral salts arising from washing with ordinary soap; increase the washing activity of surfactants; prevent the back sedimentation of suspended dirt particles on the washed surface; cheap to manufacture.
All these properties of polyphosphates make it possible to reduce the content of the more expensive main surfactant component in the CMC.
As a rule, any synthetic detergent contains a fragrance - a substance with a pleasant smell, which is transmitted by the laundry when using SMS.
Almost all CMCs contain a substance called sodium carboxymethyl cellulose. It is a high molecular weight synthetic product that is soluble in water. Its main purpose is to be, along with phosphates, an antiresorptive, that is, to prevent dirt from settling on already washed fibers.
Most of them have several advantages over soap, which has long been used for this purpose. For example, surfactants dissolve and foam well even in hard water. The potassium and magnesium salts formed in hard water do not impair the detergent effect of surfactants and do not form a white bloom on the hair.
The main active ingredients of all washing powders, the so-called. Surfactants (surfactants) are extremely active chemical compounds. Having some chemical affinity with certain components of human and animal cell membranes, surfactants, when ingested, accumulate on cell membranes, covering their surface with a thin layer and, at a certain concentration, can cause disruption of the most important biochemical processes occurring in them, disrupt the function and integrity itself cells.
In experiments on animals, scientists have found that surfactants significantly change the intensity of redox reactions, affect the activity of a number of important enzymes, and disrupt protein, carbohydrate and fat metabolism. Surfactant anions are especially aggressive in their actions. They are capable of causing gross violations of immunity, the development of allergies, damage to the brain, liver, kidneys, lungs. This is one of the reasons why strict restrictions are imposed on the use of a-surfactants (anionic surfactants) in washing powder formulations in Western Europe. In the best case, their content should not exceed 2-7%. In the West, more than 10 years ago, they abandoned the use of powders containing phosphate additives in everyday life. In the markets of Germany, Italy, Austria, Holland and Norway, only phosphate-free detergents are sold. In Germany, the use of phosphate powders is prohibited federal law... In other countries, such as France, Great Britain, Spain, in accordance with government decisions, the content of phosphates in SMS is strictly regulated (no more than 12%).
The presence of phosphate additives in powders leads to a significant increase in the toxic properties of a-surfactants. On the one hand, these additives create conditions for a more intensive penetration of a-surfactants through intact skin, promote increased defatting of the skin, more active destruction of cell membranes, and sharply reduce the barrier function of the skin. Surfactants penetrate into the microvessels of the skin, are absorbed into the bloodstream and spread throughout the body. This leads to a change physical and chemical properties the blood itself and impaired immunity. A-surfactants have the ability to accumulate in organs. For example, 1.9% of the total amount of a-surfactants deposited on unprotected skin in the brain, 0.6% in the liver, etc. They act like poisons: in the lungs they cause hyperemia, emphysema, in the liver they damage the function of cells, which leads to an increase in cholesterol and intensifies the phenomena of atherosclerosis in the vessels of the heart and brain, disrupts the transmission of nerve impulses in the central and peripheral nervous systems.
But this does not exhaust the harmful effect of phosphates - they pose a great threat to our environment. Getting after washing together with sewage into reservoirs, phosphates are taken to act as fertilizers. The "harvest" of algae in reservoirs begins to grow by leaps and bounds. Algae, decomposing, emit huge amounts of methane, ammonia, hydrogen sulfide, which destroy all life in the water. The overgrowth of reservoirs and the clogging of slowly flowing waters leads to gross disturbances of the ecosystems of reservoirs, a deterioration of oxygen exchange in the hydrosphere and creates difficulties in providing for the population. drinking water... For this reason, in many countries, the use of phosphate SMS has been legally prohibited.
The traditional disadvantage of surfactants is stiffness, which is expressed in skin irritation, dryness and discomfort after using shampoo or shower gel.
The skin of the hands, in contact with active chemical solutions of washing powders, becomes the main conductor for the penetration of hazardous chemical agents into the human body. A-surfactants actively penetrate even through intact skin of the hands and, with the assistance of phosphates, enzymes and chlorine, intensively disinfect it. The restoration of normal fat and moisture content of the skin occurs no earlier than 3-4 hours, and with repeated use due to the accumulation of the harmful effect, the lack of fatty coating of the skin is felt within two days. The barrier functions of the skin are reduced, and conditions are created for the intensive penetration of not only a-surfactants into the body, but also any toxic compounds - bacteriological toxins, heavy metals, etc. After several washings with phosphate powders, skin inflammations - dermatitis often develop. A pipeline of pathological immune responses is launched.
Bibliography
Van Krevelen D.V., Properties and chemical structure polymers, per. from English., M., 1976;
http://provisor.com
http://pharmvestnik.ru
http://darnitsa.ua
http://ru.wikipedia.org/
http://wiki.laser.ru
http://ximicat.com
For the preparation of this work were used materials from the site