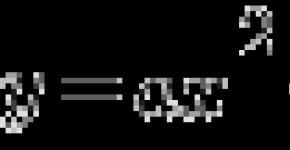Surface tension surfactant. Surfactants: General Information
Surface-active substances (Peav.) - chemical compoundswhich concentrating on the surface of the phase partition cause a decrease in surface tension.
The main quantitative characteristic of the surfactant is surface activity - The ability of the substance to reduce the surface tension at the interface between the phase separation is the derivative of the surface tension at the concentration of the surfactant when the sirry is sized to zero. However, the surfactant has a solubility limit (the so-called critical concentration of micelle formation or KKM), with the achievement of which, when adding a surfactant to a solution, the concentration on the border of the phase separation remains constant, but at the same time there is self-organization of the surfactant molecules in the volume solution (micelle formation or aggregation). As a result of such an aggregation, so-called micelles are formed. Distinctive feature Micelle formation serves as clouding of the solution of surfactant. Water solutions surfactant, with micelle formation also acquire a bluish tint (shapeless tint) due to the refraction of light with micelles.
- Methods for determining KKM:
- Surface Tension Method
- Method of measuring contact angle with TV. or liquid surface (Contact Angle)
- Rotating drop method (Spindrop / Spinning Drop)
Paving structure
Classification of Pav.
- Ionogenic surfactants
- Cationic surfactants
- Anionic Peav.
- Non-ionic surfactants
- Alkylpolyglucosides
- Alkylpolyethoxyulat
The effect of surfactants on the ecology
Pav is divided into those who quickly destroy in environment And those that are not destroyed and can accumulate in organisms in unacceptable concentrations. One of the main negative effects of surfactants in the environment is a decrease in surface tension. For example, in the ocean, the change in the surface tension leads to a decrease in the retention indicator CO 2 in the mass of water. Only a few surfactants are considered safe (alkylpolygosides), since their degradation products are carbohydrates. However, when adsorbing the surfactant on the surface of the Earth's particles / sand, the degree / speed of their degradation is reduced repeatedly. Since almost all surfactants used in industry and household, have a positive adsorption on the particles of the earth, sand, clay, under normal conditions they can release (desorbate) heavy metal ions held by these particles, and thereby increase the risk of data from the substances into the human body.
Pav in the World Ocean
According to some data, the surfactant, adsorbed on the surface of water in reservoirs, increases the absorption of the wavelength of the radar signal. In other words, radars and satellites worse capture the signal from the objects under water in reservoirs with a certain surfactant concentration.
Areas of use
- Detergents. The main use of surfactants - as an active component of detergents and cleaning products, soap, for premises, dishes, clothing, clothing, cars, etc. In 2007, more than 1 million tons of synthetic were produced in Russia. detergentsMainly - washing powders.
- Cosmetics. The main use of surfactant in cosmetics - shampoos, where the PAV content can reach tens of percent of the total volume. Also, the surfactant is used in small quantities in toothpaste, lotions, tonic and other products.
- Textile industry. Pav is used mainly to remove static electricity on synthetic tissue fibers.
- Leather industry. Protection leather Products From lung damage and sticking.
- Paint and varnish industry. The surfactant is used to reduce surface tension, which ensures the lung penetration of the colorful material into small recesses on the surface treated and filling them with displacement from the other substance (for example, water).
- Paper industry. The surfactant is used to separate ink and boiled cellulose when recycling used paper. Peat molecules are adsorbed on ink pigment. Pigment becomes hydrophobic. Then the air is passed through a solution of pigment and cellulose. Air bubbles are adsorbed on the hydrophobic part of the surfactant and the ink pigment particle is float to the surface. See Flotation.
- Metallurgy. Pav emulsions are used to lubricate rolling mills. Reduce friction. Hold high temperatures in which oil burns.
- Plant protection. Pav is widely used in agronomy and agriculture For the formation of emulsions. Used to increase the efficiency of transportation of nutrients to plants through membrane walls.
- Food industry. Pav is used in ice cream, chocolate, whipped cream and sauces for salads and other dishes.
- Oil production. The surfactant is used for the hydrophobization of the crusar zone of the formation (PPP) in order to increase oil recovery.
see also
Wikimedia Foundation. 2010.
Watch what is a "surfactant" in other dictionaries:
Surface active substance, substance that reduces the surface tension of the solvent in which it is dissolved. In water, for example, the surfaces of the active substances apply detergents and soap. Their molecules focus on ... ... Scientific and Technical Encyclopedic Dictionary
surface-active substance - Paving agent capable of adsorbing on the surface of the phase section with a corresponding decrease in their surface tension. Note The composition of magnetic suspensions use corrosion inhibitors, defoamers, stabilizers, wethers and others ...
surface-active substance - 3.19 superficial active substance: Mineral or organic additives introduced into the mixture to increase the adhesion of the binder with the surface of the stone material or in order to regulate the formation processes in the mixture. A source … Dictionary directory terms of regulatory and technical documentation
Term Surface Active Substance The term in English Surfactant Surinimonis Surfactant Abbreviations Related Amphoteric Surfactant terms, hydrophobic interaction, dispersion, colloid chemistry, critical concentration of micelle formation, ... ... encyclopedic Dictionary Nanotechnology
surface-active substance - Aktyvioji Paviršiaus Medžiaga Statusas T SritrogijaAcija Ir Metrologija apibrėžtis Medžiaga, Kuri įterpta į Skysčio AR Kietojo Kūno Paviršių Sumažina to Paviršiaus įtemptį. Atitikmenys: Angl. SURFACE ACTIVE SUBSTANCE; Surfactant Vok. ... ... Penkiakalbis AiškinaMasis Metrologijos Terminų žodynas
surface-active substance - Paviršinio Aktyvumo Medžiaga Statusas T Sritis Chemija apibrėžtis Medžiaga, Kuri AdSorbuojasi Fazių Sąlytyje Ir Sumažina Paviršiaus įtemptį. Santrumpa (OS) Pam Atitikmenys: Angl. SURFACE ACTIVE SUBSTANCE; Surfactant Rus. superficially active ... ... Chemijos Terminų aiškinamasis žodynas
surface-active substance - Aktyvioji Paviršiaus Medžiaga Statusas T Sritis Fizika Atitikmenys: ANGL. SURFACE ACTIVE SUBSTANCE; Surfactant Vok. Grenzflächenaktiver Stoff, M; Oberflächenaktiver Stoff, M; TENSID, N RUS. Surface active substance, N PRANC. Agent Tensio ... ... Fizikos Terminų žodynas
The substance capable of adsorbing on the surface of the phase section (for example, liquid and gas) and reduce its surface tension; P. a. in. Used, for example, as detergents, when obtaining water dispersions ... Big Medical Dictionary
surfactant with low foaming ability - - Themes Oil and Gas Industry EN LOW FOAMING SURFACTANT ... Technical translator directory
See Antiatelectatic Factor ... Big Medical Dictionary
Surfactants have a polar (asymmetric) structure of molecules, capable of adsorbing on the border of two environments and lower the free surface energy of the system. Completely minor additives of surfactants can change the properties of the particle surface and give the material new qualities. The action of the surfactant is based on the adsorption phenomenon, which simultaneously leads to one or two opposite effects: reducing the interaction between particles and stabilizing the surface of the section between them due to the formation of the interfacial layer. For most surfactants, the linear structure of molecules is characteristic, the length of which significantly exceeds the transverse dimensions (Fig. 15). Molecules radicals consist of groups related by its properties of solvent molecules, and from functional groups with properties, sharply different from them. These are polar hydrophilic groups, possess sharply pronounced valences and have a certain effect on wetting, lubricating and other actions associated with the concept of surface activity . This reduces the supply of free energy with heat release as a result of adsorption. Hydrophilic groups at the ends of hydrocarbon non-polar chains can be hydroxyl - it, carboxyl - coxy, amino - nn 2, sulfo - SO and other strongly interacting groups. Functional groups are hydrophobic hydrocarbon radicals characterized by side valence bonds. Hydrophobic interactions exist independently of the intermolecular forces, being an additional factor contributing to rapprochement, "sticking" of non-polar groups or molecules. The adsorption monomolecular layer of pump molecules with free ends of hydrocarbon chains is focused on
the surface of the particles and makes it unmatched, hydrophobic.
The effectiveness of this or that additive additive depends on physico-chemical properties material. The surfactant, which gives the effect in one chemical system, may not have any action or obviously the opposite - to another. At the same time, the concentration of surfactant is very important, determining the degree of saturation of the adsorption layer. Sometimes an action similar to the surfactant exhibit high molecular compounds, although they do not change the surface tension of water, for example, polyvinyl alcohol, cellulose derivatives, starch and even biopolymers (protein compounds). The action of surfactants can have electrolytes and substances insoluble in water. Therefore, it is very difficult to determine the concept of "surfactant". In a broad sense, this concept refers to any substance that in small quantities changes significantly changes the surface properties of the dispersed system.
The surfactant classification is very diverse and in some cases contradictory. Multiple attempts have been taken by classification by different features. On the Rebinder, all surfactants are divided into four groups on the mechanism:
- wetting agents, defoamers and foaming agents, i.e., active liquid-gas on the interface. They can reduce the surface tension of water from 0.07 to 0.03-0.05 J / m 2;
- dispersants, peptizers;
- stabilizers, adsorption plasticizers and dilutes (viscosity slides);
- Detergents with all properties of surfactants.
Abroad is widely used classification of surfactant for functional purposes: dilutes, wetting agents, dispersants, defloculants, foaming agents and defoamers, emulsifiers, stabilizers of dispersed systems. Aloams also binders, plasticizing and lubricating substances.
According to the chemical structure of Pav, it is classified depending on the nature of hydrophilic groups and hydrophobic radicals. The radicals are separated into two groups - ionogenic and non-ionic, the first can be anion and cationic.
Non-ionic surfactants contain non-ionizing finite groups with high affinity for the dispersion medium (water), which include usually oxygen, nitrogen atoms, sulfur. Anionactive surfactants - compounds in which a long hydrocarbon chain of molecules with low affinity for a dispersion medium is part of an anion generated in aqueous solution. For example, the coxy is a carboxyl group, SO 3 H - sulfugugroup, OSO 3 H - a group of ether, H 2 SO 4, etc. To anionactive surfactants include carboxylic acid salts, alkyl sulfates, alkyl sulfonates, etc. Cationic substances formed in aqueous solutions cations containing a long hydrocarbon radical. For example, 1-, 2-, 3- and 4-substituted ammonium and others. Examples of such substances may be salts of amines, ammonium bases, etc. Sometimes they allocate the third group of surfactant, which includes amphoteric electrolytes and ampholite substances that depend on The nature of the dispersed phase can be exhibiting both sour and basic properties. Ampholites are insoluble in water, but active in non-aqueous media, such as oleic acid in hydrocarbons.
Japanese researchers offer classification of surfactant for physico-chemical properties: molecular weight, molecular structure, chemical activity, etc. arising from the surfactant gel-like shells on solid particles as a result of various orientation of polar and non-polar groups can cause a variety of effects: discharge; stabilization; dispersion; foaming; Binding, plasticizing and lubricating actions.
The positive effect of the surfactant has only at a certain concentration. On the issue of the optimal number of imposed surfactants, there are very diverse opinions. P. A. Rebelder Indicates that for particles
1-10 microns required amount Pav should be 0.1-0.5%. Other sources are given 0.05-1% and more for different dispersion. For ferrites, it was found that for the formation of a monomolecular layer during dry oil, the surfactant must be taken at the rate of 0.25 mg per 1 m 2 of the specific surface of the initial product; For wet grinding - 0.15-0.20 mg / m 2. Practice shows that the concentration of surfactants in each particular case should be seized experimentally.
In the Ceramic Rem technology, you can select four directions for the use of surfactants, which allow you to intensify physico-chemical changes and transformations in materials and manage them in the synthesis process:
- intensification of the processes of fine grinding of powders to increase the dispersion of material and reduce the grinding time when the specified dispersion is reached;
- regulation of the properties of physicochemical dispersed systems (suspensions, shrackers, pastes) in technological processes. Here the processes of liquefaction (or lowering viscosity with increasing fluidity without decreasing moisture content), stabilization of rheological characteristics, foaming in dispersed systems, etc.;
- control of the processes of flare formation in spraying suspensions when obtaining the specified sizes, form and dispersibility of the torch sprayed;
- an increase in the plasticity of the molding masses, especially obtained when exposed to elevated temperatures, and the density of manufactured billets as a result of the introduction of a complex of binders, plasticizing and lubricating substances.
In relation to the non-polar phase (gas, hydrocarbon, non-polar surface of the solid), has a hydrocarbon radical, which is energized from the polar medium. In an aqueous solution of surfactants, an adsorption with hydrocarbon radicals, oriented to the side, is formed. As it satisfies (ions) the surfactant, compacting in the surface layer, are located perpendicular to the surface (normal orientation).
Depending on the state of surfactants, truly soluble (molecular dispersed) and colloidal surfactants are conventionally distinguished in the solution. The conventionality of such a separation is that the same surfactant may relate to both groups depending on the conditions and Him. Nature (polarity) solvent. Both groups of surfactants are adsorbed on the phase boundaries, i.e. they are expressed in solutions, while the volume properties associated with the occurrence of colloidal (micellar) phase, only colloidal surfactants exhibit. These groups of surfactants are distinguished by the value of the dimensionless value, which is called. Hydrophilic-lipophil balance (GLB) and is determined by the ratio:
where -comers (free energy interaction) of the non-polar part of the surfactant to the hydrocarbon (B-dimensionless parameter, depending on the nature of the surfactant, -svob. Energy is completed. per group CH 2, V-number CH 2 groups in a hydrocarbon radical), a -Ofness of the polar group to. For colloidal surfactants (b + or, where M indices correspond to the minimum affinity values \u200b\u200bin which the colloid properties of surfactants begin to manifest. The minimum number of carbon in the radical for different species Colloidal surfactants lies within 8-12, i.e. Colloidal surfactants have a fairly large hydrocarbon radical. At the same time, colloidal surfactants should have a true solubility in, i.e. The polarity of the hydrophilic group should also be high enough. This corresponds to the condition:
![]()
In the beginning. 60s. 20 V. D. Blisis was developed by the GLB scale with values \u200b\u200bfrom about 40. The surfactant with lipophilic properties have low GLB values, with hydrophilic-high. Each group included in the surfactant is attributed to the group number. When adding these numbers, GLB is obtained by the formula:
GLB \u003d hydrophilic group numbers + 4-hydrophobic group numbers + 7.
Although the concept of GLB is quite formal, it allows you to determine the scope of application of surfactant. So, for the formation of water / GLB oil lies in the range of 3-6, oil / in Da-8-16, for wetting agents-7-9, for funds-13-15.
Amphoteric (ampholite) surfactants contain a hydrophilic radical and a hydrophobic part capable of being an acceptor or donor depending on the pH of the solution. Typically, these surfactants include one or more of the main and acidic groups, may also contain a non-ionic polyglycolic group. Depending on the size of the pH, they exhibit the properties of cationic or anionactive surfactants. For some pH values, called. The surfactant exist in the form of zwitter ions. The ionization constants of acidic and basic groups of truly soluble amphoteric surfactants are very low, but most often encountered cation-oriented and anion-oriented zwitter ions. As a cationic group, the primary, secondary or tertiary ammonium group, the remainder or is usually served as a cationic group. In principle, instead of n m. B. S, P, AS, etc. Anionic groups are carboxyl, sulfonate, sulfoester or phosphate groups.
By chemical The structure and some similarity of the properties of ampholite PAV are divided into 5 land. Groups: 1) Alkylaminocarboxylic acids RNH (CH 2) N Cooh; Alkyl radical is usually normal (straight-chain), but if it is located between the amine group and carboxyl, sometimes has a branched character. The same group includes alkylamino-phenylcarboxylic acids RNHC 6 H 4 COOH; alkylaminocarboxylic acids with primary, secondary or tertiary amino group RCH (NH 2) COOH, RCH (NHR) COOH, R (CH 3) NCH 2 COOH; with an intermediate hydroxyl, essential, ester, amide or sulfoamide group; Substances with two or more amino and amidogroups, with several amino and hydroxyl groups.
2) Alkylbetains are the most important group of Zwitter-ion surfactant. They can be divided into 5 axes. Groups: a) alkylbetaine -С-alkylbetainins RCH COO - and N-alkylbetainins Rn + (CH 3) 2 CH 2 co -; b) sulfite, sulfate, sulfate and phosphantbetainins Rn + (CH 3) 2 CH 2 CH 2 RN + (CH 3) 2 CH 2 CH 2, RC 6 H 4 CH 2 N + (CH 3) 2 CH 2 CH 2 RN + (CH 3) 2 CH 2 CH (OH) CH 2 OP; c) Amidobetains RCONH (CH 2) 3 N + (CH 3) 2 Coo -; d) oxytelationed Rn + [(C 2 H 4 O) P H] [(C 2 H 4 O) G H] CH 2 Coo -; e) Dr. Zwitter-ion surfactant.
3) derivatives of alkylimidazolins, in which anionic and cationic groups have approximately the same ionization constants (formulas VII and VIII), where R-alkyl C 7 -C 17, R "-H, Na, CH 2 Coom (M-Metal). By The structure and methods of synthesis are isolated by betaine surfactants, including carboxy, sulfo-, sulfate or sulfoeher group [formula IX; R "\u003d (CH 2) N COO -, (CH 2) 3, CH 2 CH (OH) CH 2] and other ("unbales") imidazoline surfactants [formula x; R "\u003d CH 2 Coona, (CH 2) 2 N (CH 2 COOH) 2, (CH 2) 2 n \u003d CHC 6 H 4 SO 3 H, (CH 2) 2 OSO 3 H]. Balanced by ionized groups provides These compounds are good colloid-chemical and sanitary and hygienic properties.
4) Alkylaminoalkonates and sulfates (AAAC 1 and AAAC 2 acc.). Anion and landmark. The substances easily pass into the zwitter-ion form, which allows them to be separated in its pure form. The ionization constant of the acid group is much larger than the main one, so they are used in alkaline environment. However, in the case of several basic groups and, if there is a similar to the acid group, other hydrophilic groups, these substances according to properties and applications are similar to ampholite surfactants and have a bactericidal action. Depending on the ionization constants, AAAC 1 Rn (R ") can be distinguished by AAAC 1 Rn (R") - R: -SO 3 M, AAAC 2 Rn (R ") - R: - OSO 3 m, derivatives of aromatic amino unulfonic acid RR" N-AR-SO 3 m, Amineosulfonates with N in heterocycles (formula XI); aminophosphates, aminophosphonates and other amine-containing compounds of type RR "R: P (O) (OH) 2, RR" R "" OP (O) (OH) 2, where R and R " -Dine and short hydrocarbon radicals, R: -corrotine bivalent radical; Seda. Rn (CH 2 CH 2 SO 3 Na) 2. Their difference is good ability to disperse calcium and resistance to.
5) Polymer ampholite surfactants: natural (proteins, nucleic acids, etc.); Modified natural (oligomeric hydrolyzates, sulfatim. Hitin); Step, fatty acids; derivatives obtained by the introduction of carboxyl and diethanolamine ethyl groups; synthetic in which combined structural features All the above groups of amphoteric surfactants (see, for example, formula XII-XVI).
Application surfactant.The world production of Pav is 2-3 kg per capita per year. Approximately 50% of the surfactants produced is used for (detergent and cleaning products, cosmetics), the rest, in industry and with. x-ve. Simultaneously with the annual increase in the supply of surfactants, the ratio between their use in everyday life changes in favor of industry.
The use of surfactants is determined by them, the structure of the adsorption layers and the volume properties of solutions. The surfactants of both groups (truly soluble and colloidal) are used as dispersants with, drilling solid rocks (hardness lowrapers), to improve, decrease and wear, intensity of reservoir oil recovery, etc. An important aspect of using surfactants is the formation and destruction ,. The widespread use of PAV is found for regulation and stability with a liquid dispersion medium (water and organic). The Mi-Cell systems formed by the surfactant are widely used both in the water and non-aqueous environment for which the surface activity of surfactants and not the properties of their adsorbz are important. Layers, and volume properties: sharply pronounced anomalies with an increase in surfactant right up to education, for example in an aqueous medium, crystallizer. structures of solid or firm-shaped structures (based on oil oils).

Pav is used by more than 100 industries national economy. Most of the surfactants produced are used as part of the CP-B, in the production of tissues and products based on synthetic. And Pri. fibers. Close-in consumers of surfactants include oil, chemical. Industry, industry is building. Materials and a number of others. Most important applications surfactant:
Drilling with clay solutions and reversible water / oil. To regulate the aggregative stability and the rheological characteristics of solutions, high molecular weight surfactants, poly-acrylamide, etc., are used in calcium pries. and synthetic. fatty acids (C 16 -C 18 and higher), alkylomatic. , alkylamines, alkylamidoamines, alkylimidazolins;
Increased reservoir oil recovery through micellar factoting (oxytentive and, alkylaromatic. Sulfonates);
Antioxidative, anti-faded and other additives in the production of Miner. Oils (soap synthetic. fatty acids, petroleum, oxyelectr. Alcohols) and plastic. lubricants (derivatives, arylamans, alkyl and aryl phosphates);
Regulation with iron and manganese (soaps Pri. And synthetic. Fatty acids, higher aliphatic. Amines), rare (alkylarson and alkyl phosphonic acids, alkylaromatic sulfonates);
Emulsion, obtaining, etc. Vinyl (carboxymethyl cellulose, poly, synthetic. Fatty acids, alkyl sulfates, and alkylphenols);
Him production. fibers (oxyteric. and amides, and higher and acids);
Mechanical restoration
Surfactants - compounds that affect the magnitude of the surface tension. In the process of interaction of fluid molecules between them, clutch forces are formed. This force will be different in surface and internal (deep) layers. Considering the condition of the fluid, it is easy to establish that the particles that are directed inside the system, with of different side Surrounded by the same molecules that affect them. Equality of all forces that act on such a molecule is zero. Therefore, fluids have the smallest surface at a given volume. This is clearly manifested in a spherical form of drops. The presence of impurities of various compounds in liquids causes the magnitude of the surface tension.
The structure of Peav Molecules
Particles of fatty acids and alcohols consist of two parts that have different properties, so these compounds are very often called dilophilic structures. One part of the molecule is represented by a hydrocarbon chain, and the other - by different functional groups (amino group, hydroxyl, carboxyl, sulfoguroup). The longer the hydrocarbon chain, the stronger the particles will be expressed, they weaker will interact with water.
Surfactants (surfactants) of organic origin: proteins, soap, alcohols, ketones, aldehydes, tannides, ketones, etc. Surfactants do not affect surface tension (starch, glucose, fructose).
Non-ionic surfactants (NPAV) are high molecular weight bios compounds that in water do not form ions. In the reservoirs, these substances come together with industrial (chemical, textile, household (the use of diverse in everyday life) drains, as well as with waste waste from agricultural land (herbicides, fungicides, insecticides, as well as foliants as emulsifiers).
Surfactants: harm and benefits
Surface tension has great value For suction processes in the intestine. For example, fats, as well as lipids in the food tract come in the form of droplets. The latter are emulsified in the small intestine with bile acids. Only after that the specified fats are hydrolyzed by lipolytic enzymes. Very often, soap (surfactants) is added to increase the performance of the insecticides. The manipulation carried out allows insecticides to interact better with the surface of the insect body. However, surfactants have not only positive, but also a negative impact on the body. For example, the shampoo includes very harmful foaming agents (surfactant), such as sodium and ammonium lauril sulfate, ammonium and sodium lauret sulfate. There is an opinion that these components have a carcinogenic effect.