Aqueous ammonia solution formula. Properties, application and storage of aqueous ammonia.
Ammonia water has found quite a wide application in, and this is primarily due to its low cost and ease of use. Today, chemical plants produce two grades of this substance. Brand "A" is used for various industrial needs, and brand "B" is used in agriculture. The latter will be discussed in this article.
Description and composition
Simply put, ammonia water is a solution of ammonia in water. Outwardly, it is a clear liquid, which sometimes may have a yellowish tint. It has a sharp specific aroma, reminiscent of the smell of rotten eggs.
Did you know? A 10% ammonium solution has found wide application in medicine and is called " ammonia».
The chemical formula of this substance is NH4OH
. The percentage of ammonia in this solution is usually about 30%: 70% is water, and about 24.6%.  In order to obtain such a solution, coke-chemical or synthetic ammonia is dissolved under a pressure of 2 atmospheres. We also advise you to learn how to properly apply in gardening and horticulture. Ammonia is highly volatile and can erode out of solution if stored improperly. Therefore, under adverse conditions, it may well be unusable. The density of ammonia water is about 0.9 g per 1 cu. cm.
In order to obtain such a solution, coke-chemical or synthetic ammonia is dissolved under a pressure of 2 atmospheres. We also advise you to learn how to properly apply in gardening and horticulture. Ammonia is highly volatile and can erode out of solution if stored improperly. Therefore, under adverse conditions, it may well be unusable. The density of ammonia water is about 0.9 g per 1 cu. cm.
Impact on the garden
Ammonia water is actively used for the garden, which is associated with its low cost and ease of use. For example, the price of a liter of this solution starts from 10 rubles per kg, while a kilogram of ammonium nitrate costs at least 25 rubles.  Ammonia-based fertilizer is suitable for almost any crop, which makes it one of the most sought after and widely used on the market.
Ammonia-based fertilizer is suitable for almost any crop, which makes it one of the most sought after and widely used on the market.
On the ground
The use of this fertilizer is relevant on the most various types soils. It should always be remembered that this substance has alkaline reaction, in connection with which it is able to change .
The best effect was recorded when applied to well-cultivated land and which contains a large amount.  A similar effect is obtained due to the fact that in such a process of absorption of ammonia is much more intense than on poor and light soils, which, in turn, indicates that plants absorb much more nitrogen, which is part of ammonia water.
A similar effect is obtained due to the fact that in such a process of absorption of ammonia is much more intense than on poor and light soils, which, in turn, indicates that plants absorb much more nitrogen, which is part of ammonia water.
Did you know? Nitrogen, the main component of ammonia,- one of the most common elements on Earth and the main component of air (78.09%).
On dry soil and soil with a light mechanical composition, the efficiency of ammonium hydrate will be somewhat lower due to its high volatility. Ammonia simply evaporates from the treated area if it is not repaired to a sufficient depth.  When using ammonia water on cohesive soils that are highly resistant to erosion and particle breakdown (e.g. loam), special considerations should be taken. temperature regime, since high temperatures will contribute to the rapid disintegration of the molecules of the substance.
When using ammonia water on cohesive soils that are highly resistant to erosion and particle breakdown (e.g. loam), special considerations should be taken. temperature regime, since high temperatures will contribute to the rapid disintegration of the molecules of the substance.
The optimal application period will be early spring, when the average daily temperature still does not exceed 10 ° C. Find out what is best for your plants -.
For culture
In an extremely favorable way, the use of ammonium hydrate will affect crops for which an increased protein content is a positive property, for example, for. This is due to the fact that ammonia increases the concentration of this substance in plants.  Ammonium hydrate, like any other nitrogenous, contributes to the intensification of photosynthesis processes in plants and increases the set of green mass. Find out what are the ways In this regard, it is extremely important to comply with the application rates, since there is a possibility of getting a low yield, but at the same time with a fairly intense stem and leaves.
Ammonium hydrate, like any other nitrogenous, contributes to the intensification of photosynthesis processes in plants and increases the set of green mass. Find out what are the ways In this regard, it is extremely important to comply with the application rates, since there is a possibility of getting a low yield, but at the same time with a fairly intense stem and leaves.
Important! Do not allow the solution to come into contact with the plants, as this can damage and even completely kill the plant.
Methods and application rates
Self-treatment with ammonia water is not a tricky business. It is enough to simply irrigate the selected plots of land with a solution at a depth of 10 cm on heavy soils and about 15 cm on light soils. This technique is generally accepted in and is called "fertigation".
Important! Fertigation will be extremely inefficient in hot weather due to the abundant evaporation of the active substance.
The best time for such processing is the autumn period, about six months before the start of the active summer season.  But fertilization is not excluded in the spring as part of complex measures to prepare for sowing.
But fertilization is not excluded in the spring as part of complex measures to prepare for sowing.
Now it is worth saying a few words about the application rates:
- In the event that plants are planted in narrow rows or the land intended for planting crops is fertilized, ammonium hydrate is poured using specialized equipment. The spacing between coulters is approx. 25–30 cm, and the amount of water required for 1 ha - about 50 kg.
- Processing large areas where landing is planned vegetable crops, fertilizer is applied between the rows. Norms - about 60 kg per 1 ha.
- When using ammonia water for industrial crops, it should be remembered that the norms are slightly increased - up to 70 kg per 1 ha.
 High concentrations of ammonium in the air can cause nausea, dizziness, disorientation, abdominal pain, coughing and choking. If such symptoms occur, it is worth immediately stopping the treatment and leaving the area saturated with ammonia vapor. The main "competitor" of ammonia water is urea, which contains almost twice as much nitrogen. In case of contact with skin or mucous membranes, it is recommended to wash them with plenty of clean boiled water and if complications arise, seek medical attention.
High concentrations of ammonium in the air can cause nausea, dizziness, disorientation, abdominal pain, coughing and choking. If such symptoms occur, it is worth immediately stopping the treatment and leaving the area saturated with ammonia vapor. The main "competitor" of ammonia water is urea, which contains almost twice as much nitrogen. In case of contact with skin or mucous membranes, it is recommended to wash them with plenty of clean boiled water and if complications arise, seek medical attention. Storage features
Containers for storing ammonium hydrate can serve as steel tanks with hermetic properties, as well as fuel tanks. Ammonia water is often delivered by the manufacturer in special tanks, which must be returned after a certain period.  If you intend to store ammonium hydrate on your own, be aware of its volatile properties and look for containers that have good sealing properties, otherwise the full potential of this fertilizer will simply evaporate.
If you intend to store ammonium hydrate on your own, be aware of its volatile properties and look for containers that have good sealing properties, otherwise the full potential of this fertilizer will simply evaporate.
This fertilizer, despite the small danger it poses, is perfect for any gardener, both with experience and a beginner.
By observing all precautions, you will undoubtedly derive great benefit from the use of this substance. Good luck to you and your garden!
Was this article helpful?
Not really
And hydrogen. It is a colorless gas, but with a pungent odor. Chemical composition reflects the formula of ammonia - NH 3. An increase in pressure or a decrease in the temperature of a substance leads to its transformation into a colorless liquid. Gaseous ammonia and its solutions are widely used in industry and agriculture. In medicine, 10% ammonium hydroxide is used - ammonia.
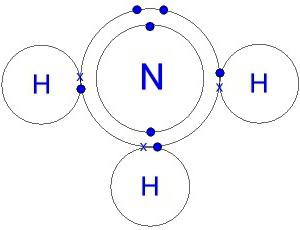
The structure of the molecule. Electronic formula of ammonia
The hydrogen nitride molecule is shaped like a pyramid, at the base of which is nitrogen bonded to three hydrogen atoms. The N–H bonds are strongly polarized. Nitrogen attracts the bonding electron pair more strongly. Therefore, the negative charge is accumulated on the N atoms, while the positive charge is concentrated on the hydrogen. An idea of this process is given by the model of the molecule, electronic and ammonia.
Hydrogen nitride is very soluble in water (700:1 at 20°C). The presence of practically free protons leads to the formation of numerous hydrogen "bridges" that connect the molecules to each other. Structural features and chemical bonding also lead to the fact that ammonia is easily liquefied with an increase in pressure or a decrease in temperature (-33 ° C).

origin of name
The term "ammonia" was introduced into scientific use in 1801 at the suggestion of the Russian chemist Y. Zakharov, but the substance has been known to mankind since ancient times. A gas with a pungent odor is released during the decay of waste products, many organic compounds, for example, proteins and urea, during the decomposition of ammonium salts. Historians of chemistry believe that the substance was named after ancient egyptian god Amon. The oasis of Siwa (Ammon) is located in North Africa. The ruins are preserved in the surroundings. ancient city and a temple, next to which there are deposits of ammonium chloride. This substance in Europe was called the "salt of Amon." There is a legend that the inhabitants of the Siwa oasis sniffed salt in the temple.

Obtaining hydrogen nitride
The English physicist and chemist R. Boyle burned manure in experiments and observed the formation of white smoke over a stick dipped in hydrochloric acid and introduced into the stream of the resulting gas. In 1774, another British chemist, D. Priestley, heated ammonium chloride with slaked lime and isolated a gaseous substance. Priestley called the compound "alkaline air", because its solution showed properties. Boyle's experiment, in which ammonia interacted with hydrochloric acid, was explained. Solid white color occurs when the molecules of the reacting substances come into contact directly in the air.
The chemical formula of ammonia was established in 1875 by the Frenchman C. Berthollet, who conducted an experiment on the decomposition of a substance into its constituent components under the action of an electric discharge. Until now, the experiments of Priestley, Boyle and Berthollet are being reproduced in laboratories to obtain hydrogen nitride and ammonium chloride. The industrial method was developed in 1901 by A. Le Chatelier, who received a patent for a method for synthesizing a substance from nitrogen and hydrogen.
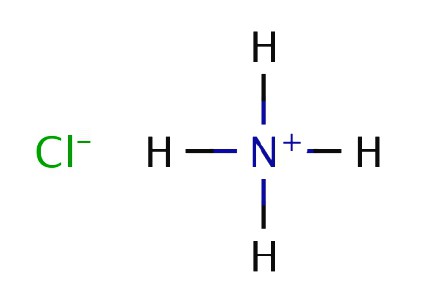
Ammonia solution. Formula and Properties
An aqueous solution of ammonia is usually written as hydroxide - NH 4 OH. It exhibits the properties of a weak alkali:
- dissociates into ions NH 3 + H 2 O \u003d NH 4 OH \u003d NH 4 + + OH -;
- colors the solution of phenolphthalein in crimson color;
- reacts with acids to form salt and water;
- precipitates Cu(OH) 2 as a bright blue substance when mixed with soluble copper salts.
The equilibrium in the reaction of the interaction of ammonia with water is shifted towards the starting materials. Preheated hydrogen nitride burns well in oxygen. Nitrogen is oxidized to diatomic molecules a simple substance N2. Ammonia also exhibits reducing properties in reaction with copper (II) oxide.

The value of ammonia and its solutions
Hydrogen nitride is used in the production of ammonium salts and nitric acid, one of the most important products of the chemical industry. Ammonia serves as a raw material for the production of soda (according to the nitrate method). The content of hydrogen nitride in an industrial concentrated solution reaches 25%. In agriculture, an aqueous solution of ammonia is used. The liquid fertilizer formula is NH 4 OH. The substance is directly used as a top dressing. Other ways to enrich the soil with nitrogen are the use of salts of chlorides, phosphates. In industrial conditions and agricultural premises, it is not recommended to store mineral fertilizers containing ammonium salts together with alkalis. If the integrity of the packaging is violated, the substances can react with each other with the formation of ammonia and its release into the indoor air. The toxic compound adversely affects the respiratory organs, central nervous system person. The mixture of ammonia with air is explosive.
ammonia water
The modern chemical industry offers a large number of liquid nitrogen fertilizers, the most common of which is ammonia water. The cost of a unit of active ingredients in ammonia water is 1.5-2 times cheaper than in ammonium nitrate. In addition, as production tests have shown, labor costs for its application are reduced by a factor of three, because there is no need to prepare fertilizers for application, and all operations for use (for loading, unloading, applying) are fully mechanized. That is why ammonia water is widely used in domestic agricultural enterprises. However, it also has certain features that should not be forgotten. Ammonia water (technical water ammonia) and NH 4 OH is a 25% solution of ammonia in water. A colorless or yellowish liquid with a pungent odor of ammonia contains 20.5% nitrogen (second grade - 16-18%), it can be used for any soil and all crops with obligatory zaboro in the soil to a depth of 10-15 cm. In the USSR in 80 In the 1990s, almost 40% of nitrogen fertilizers are applied to the fields in the form of ammonia water. In the USA, up to 50% of nitrogen fertilizers are applied in liquid form. In Ukraine, only about 15% of households use ammonia water. This is due to the lack of machinery and equipment for storing, transporting and applying it to the soil.
Ammonia water is used for fertilizing cultivated crops as a nitrogen fertilizer. First, it increases the protein content in plants. Perform situations when plants need an excess of protein (for example,). Ammonia water (like any nitrogen fertilizer) also stimulates the growth of green mass. Therefore, it is important not to overdo it with the application rates of the drug: with an excess of ammonia water, you can easily get an excessive amount of green mass and less grain. It is very important that ammonia water gets into the soil, but not into the zone of the root system and not into the zone of seed germination, but deeper than the seeds or away from it, between the rows.
If you just pour the soil with a solution, then there will be large losses of ammonia through evaporation and the money will be thrown to the wind. So, ammonia water must be earned into the soil. It is desirable that the soil be moist at the same time.
Ammonia water can overwhelm the plant, scorch its roots for a short time between application and sowing, or failure to maintain application depth. However, if ammonia water is introduced in advance, roughly speaking, six months before sowing, for example, it was introduced in October, sown in March, then in principle there can be no negative impact. During spring application you need to remember a few rules: apply 1-2 weeks before sowing a crop, immediately before sowing a crop, or simultaneously with sowing.
Ammonia water is a very good fertilizer. Due to its liquid form, it can be applied very accurately, evenly, to a certain depth and over large areas.
If we talk about the effects, then ammonia water affects the harmful entomofauna, in particular, it suppresses and repels soil insects. If you add ammonia water below the laying of the seed bed, then for some time it repels soil pests that destroy the seed that germinates. Ammonia water is alkaline and can affect the acidity of the soil.
The best way to apply to the soil is emphatic, in particular in the fall. Fertilizer can be applied both for autumn plowing and in spring for pre-sowing cultivation. Suitable for all soils and all crops. Ammonia water is also used for fertilizing tilled crops.
It is mainly used separately from conventional (dry) fertilizers. According to some reports, the effect of ammonia water on winter rye, barley, potatoes, and corn is similar to that of ammonium nitrate.
With the use of ammonia water there are several difficulties. The concentration of N in ammonia water is approximately 22%, in urea - 45, in saltpeter - 34.6%. It is noticeable that ammonia water has a low ammonium content. Due to the fact that it is still liquid, it occupies a certain volume. In order to apply ammonia water, farms must have special means, equipped for the transportation of aggressive agents, which is ammonium. Also, the household should be good, quality unit for the introduction of ammonia water.
Ammonia water has peaks of its prices and a period of their fall. When the time for the introduction of nitrogen fertilizers ends, the prices for its derivatives, naturally, decrease. At this moment, it is advisable to buy ammonia water, the shelf life of which, by the way, is limited. This means that the economy needs to have a warehouse where the product will be located within 2-3 months. It must be a sealed, stable container.
Equipment for storage of ammonia water requires compliance with a lot of special permits: ecology, sanitation, etc. Accordingly, it is necessary to cooperate with these services. You need to "be friends" with the manufacturer. They buy a tank at the factory, then they bring it, then you need to download the product, transport it and save it. The rolling stock is the property of either the plant or railway, so there are deadlines for unloading. You can’t keep the tank for a long time, because penalties will be immediately issued upon the return of the container. Therefore, the farm needs special transport, storage, and machines for introducing ammonia water.
Within a radius of more than 30 km from suppliers, provision should be made for intermediate storage in railroad and (or) deep warehouses. Cargo transportation ammonia wateron railway warehouses they are delivered in railway tanks with lower and upper hatches. Ammonia water is pumped from the rail tank cars to the railroad stock tanks by a storage pump. In the absence of a railroad train, the motor pump is pumped directly into tank cars. From railroad trains to deep warehousesare transportingammonia water by road or tractor transport.
Ammonia water is stored in steel tanks that are hermetically sealed. It is allowed to use tanks from tractor fuel. Ammonia water is often delivered to the field by tractor trailer tanks (8-10 m 3), equipped for forced filling of cultivators through hermetic easily replaceable couplings. To reduce the space of vehicles, it is recommended to install field tanks of 25-50 m 3 and fill cultivators simply from these tanks. Various cultivators are used to introduce ammonia water.
A competitive analogue is UAN (carbamide-ammonia mixture), where nitrogen is contained in three forms. UAN has buffer properties (the ability to maintain the acidity of the solution even in the case of adding substances with an acidic or alkaline reaction, of course, within certain limits). Due to this property UAN can be used in tank mixes with pesticides. Regarding the minuses, it is worth noting the higher cost than in ammonia water, as well as the ability to cause corrosion of metals (stainless steel, ceramics, polymers are stable).
Everyone who works with ammonia water should be well aware of the main signs, safety requirements and strictly adhere to the rules for storing, transporting and applying ammonia water to the soil.
Ammonia water, introduced for spring cultivation in the amount of 90 kg of active ingredient per 1 ha, in the same year provided a yield of 32.2 q of stems, 6.5 q of fiber and 3.4 q of seeds per 1 ha.[ ...]
Ammonium nitrate Ammonium sulfate Urea Ammonia water Liquid ammonia Sodium nitrate Calcium nitrate Ammonium chloride Calcium cyanamide etc.[ ...]
Ammonia water is introduced into the peat pile and after that the peat is collected in caravans (piles) of 1000 or more tons each. In these heaps, peat is composted for 2-3 months, during which the ammonia nitrogen introduced into the peat is partially converted into the nitrate form of nitrogen under the influence of nitrifying microorganisms. Ready-made peat-mineral ammonia fertilizer according to a given technology for its manufacture due to the introduced mineral fertilizers should have in its composition 0.4 "% of assimilable mineral nitrogen, 0.4% P2O5 and 0.4% KrO. However, the actual content of assimilable nitrogen in practice is often is lower. This is mainly due to the fact that in the process of ammonization of peat ammonia is lost due to its volatilization. In addition, losses of nitrogenous compounds from peat heaps due to their leaching by atmospheric precipitation are possible. But even if we assume that there is no loss of nitrogen and other nutrients for plants, substances from TMAU will not occur, then, in order to introduce the average commonly used rate of active elements under such a crop as, for example, potatoes - 60 kg of nitrogen, 60 kg of P2O5 and 60 kg of KrO, you will require 15 tons of TMAU. Usually such doses are recommended TMAU: for potatoes and other tilled crops (corn, sugar beet) - 15-20 tons, for vegetables - 30 tons and for flat - 10 t.[ ...]
Ammonia - ammonium sulfate, ammonium chloride, liquid ammonia, ammonia water, carbon ammonia.[ ...]
Ammonia sulfite cyanide citrate solution: to 325 ml of ammonia water (d ° - 0.880) add 30 ml of KCl solution, 60 ml of Na23205 solution, 20 ml of ammonium citrate solution and dilute with water to 1 liter. The solution can be stored for several months.[ ...]
Ammonia water, containing impurities of ammonium carbonates, is continuously discharged into a collector, from where it is pumped to the carbamide shop for the production of liquid fertilizers. The same absorber for cleaning is supplied with ammonia purges from the safety valves of the apparatus, from the ammonia evaporator, collectors, urea solution and ammonium salts.[ ...]
Ammonia water is a solution of synthetic or coke-chemical ammonia in water, available in two grades. The first grade contains 20.5% nitrogen (25% ammonia), the second 16.4% nitrogen (20% ammonia). Coke-chemical aqueous ammonia, in addition, contains hydrogen sulfide and minor amounts of phenols, thiocyanate, cyanide and some other compounds.[ ...]
Ammonia water can be used as part of the main fertilizer in the spring, a few days before sowing spring crops; in the summer in top dressing row crops; before sowing winter crops; in a busy fallow and under autumn plowing. Fertilizer is planted with special machines on light soils to a depth of 12-16 cm and on heavy soils to a depth of 8-12 cm. The introduction of high doses of aqueous ammonia in the fall on light sandy loamy soils is associated with the possibility of ammonia losses, because part of the ammonium is not absorbed by the soil.[ ... ]
Ammonia water is a good fertilizer for potatoes, beets, cereals and vegetables. As an example, we can cite the results of four years of VIUA experiments, in which the effect of aqueous ammonia and ammonium nitrate on the potato crop was compared (Table 53).[ ...]
Ammonium nitrate. . Ammonia water.[ ...]
Ammonia water was introduced during autumn plowing under spring wheat on an area of 10 hectares. Fertilizer consumption per 1 ha - 280 kg, embedding depth -25 - 27 cm (on the bottom of the furrow - in a trickle).[ ...]
Influence of ammonia water on cabbage yield (c/ha).[ ...]
When ammonia water is introduced into the soil, ammonia is adsorbed by colloids and therefore moves weakly in it. Over time, ammonia nitrogen nitrifies and then acquires greater mobility, migrating with the soil solution. Compared to liquid ammonia, the use of ammonia water as a fertilizer is technically simpler and safer, but its major disadvantage is its low nitrogen content, which increases the costs associated with transportation, storage and application of fertilizer to the soil. Therefore, the use of ammonia water is advisable only in farms located near enterprises producing this fertilizer.[ ...]
Efficiency of ammonia water for spring wheat.[ ...]
Top dressing of hemp with ammonia water should be carried out in the phase of formation of three pairs of leaves. Feeding in the later phases of development significantly reduces its effectiveness.[ ...]
Its solubility in water is greater than that of all other gases: one volume of water absorbs about 700 volumes of ammonia at 20 ° C, a 10% ammonia solution goes on sale under the name "ammonia". 18 - 20% solution is called ammonia water and is used as a fertilizer. Liquid ammonia is a good solvent for most organic and inorganic compounds.[ ...]
The data show that ammonia water is an effective nitrogen fertilizer for spring wheat as well: an increase in yield of 2.8 centners per hectare was obtained.[ ...]
The orientation towards the use of ammonia water (on average 20.5% > 1) as a liquid nitrogen fertilizer, and not liquid ammonia (82% N) in our country, was associated with the need to make it as easy as possible to comply with safety requirements during storage, transportation and applying these fertilizers to the soil. The use of liquid nitrogen fertilizers makes it possible to fully mechanize the work of loading, transporting and applying them to the soil. At the same time, loading and unloading operations, transportation and storage are carried out according to a closed, fully sealed cycle (pump - pipe - tank - storage tanks). At the same time, labor productivity in agriculture is growing sharply. In addition, the losses of solid fertilizers that occur during their transportation, storage and application to the soil are almost eliminated with the transition to liquid fertilizers. According to VIUA, when switching to the production and use of aqueous ammonia, the total costs are reduced by more than three times compared to the costs of solid fertilizers.[ ...]
Corrosion in ammonia desorption towers from acid-free ammonia water is negligible, which makes it possible to use steel or cast iron equipment. With a high content of ammonia in the purified gas, the process begins to be seriously affected by the heat released during absorption. To ensure isothermal absorption, this heat must be removed. One of the possible solutions is the circulation of ammonia water in the absorber-cooler system, although it allows heat to be removed, it does not provide isothermality and, as noted above, leads to significant losses of ammonia as a result of violation of the counterflow principle.[ ...]
Example 7. Determine the limiting concentration of ammonia water, which can be obtained by passing a gas containing 3% of the mass of ammonia through a nozzle irrigated with water. The pressure in the scrubber is 2 atm. The solubility of ammonia in water under these conditions is characterized by Henry's law: P = 2000 X mm Hg (where X is the molar fraction of ammonia in solution).[ ...]
Most of the water used in enterprises is returned to circulation. The main effluents are ammonia water from coking plants, blowdown from the circulating cycles of wet gas cleaning of blast furnaces and oxygen converters, and effluent from the cold rolling mill.[ ...]
Given that the main raw material for the production of ammonia water is synthetic ammonia, the efficiency of ammonia water production depends entirely on the economics of ammonia production.[ ...]
The effectiveness of nitrogen fertilizers, especially ammonia water, increases if they are applied together with superphosphate. Thus, the yield of corn silage mass from 105.9 centners (third option) rose to 127.4 centners, or by 36.2 centners per hectare. And with the joint application of superphosphate with ammonium sulfate, the increase in yield was 26.3 centners per hectare (29.9%). However, it should be noted that the increase in dry matter yield under the action of ammonia water and, in particular, under the influence of ammonium sulfate, was significantly lower compared to the increase in maize silage mass.[ ...]
Data tab. 3 show that the best way the introduction of ammonia water is a combination of pre-sowing application with top dressing during the growing season. In this case, the increase in the yield of silage was 106.5 centners per hectare, while at the same rate of aqueous ammonia applied before sowing, the increase in yield was 75.5 centners per hectare.[ ...]
Addition containing molybdenum industrial waste to ammonia water is not the only way to use them. They can be used for the manufacture of liquid complex fertilizers. Molybdenum waste is suitable for the production of molybdenized superphosphate and nitrophoska.[ ...]
In the experiment of 1862, the action of urea was compared with the action of ammonia water (22'>) and ammonium sulfate (21%) on spring wheat. The experiment was carried out on gray forest heavy loam in the 4th field of the seed-growing crop rotation. Ammonia water for spring wheat was applied under the plow in autumn, and urea and ammonium sulfate in the spring for cultivation at the rate of 60 kg of nitrogen per hectare. Plots of 800 sq. m were laid in the triple gtov-! sr1.ost. The spring homonym Rmotmyam m L> was sown; April 23, seeding rate 200 kg per hectare.[ ...]
For the construction of deep warehouses for the temporary storage of ammonia water in agricultural conditions, standard projects warehouses with a capacity of 50 m3 with the installation of two tanks of 25 m3 each (Project No. 24-117-U1) and a capacity of 100 m3 also with the installation of two tanks of 50 m3 each (Project No. 24-117-U).[ ...]
V last years we studied the action of ammonium chloride (Moss), ammonia water (M) and ammonia carbonate No. 4 (compared with the action standard forms nitrogen fertilizers against the background of RK (Table 6).[ ...]
Ammonium carbonate [(GSh4)2SO3] is a crystalline product. It is obtained by saturating ammonia water with carbon dioxide, followed by distillation of ammonium carbonate at a temperature of 70-80 ° or as a result of the interaction of gaseous ammonia and carbon dioxide in the presence of water vapor. Ammonium carbonate is very unstable. In the open air, it decomposes with the release of ammonia and turns into ammonium bicarbonate. The technical product contains 21-24% nitrogen and is a mixture of ammonium carbonate [(N114)2003], ammonium bicarbonate (]MH4HC03) and ammonium carbamate.[ ...]
On the basis of the above data on ordinary medium-thick and thick chernozems, ammonia water and synthetic urea can be recommended for corn along with ammonium nitrate and ammonium sulphate. It is advisable to use ammonia water for feeding corn and on Ciscaucasian chestnut-calcareous soils.[ ...]
At the Erastov Experimental Station (author of the article and post-graduate student F. L. Moskalenko), the effect of ammonia water on the growth, development and yield of corn was studied under different terms application: simultaneously with sowing, during the period of autumn soil preparation and for pre-sowing cultivation of plough land. At the same time, some features of the effect of this fertilizer on the germination and field germination of corn seeds were revealed. Ammonia water, introduced at a rate of 30 kg of nitrogen per 1 ha in the same row as the seeds, to a depth of 10 cm on the day of sowing, reduced the field germination of seeds by 80-90%, and with an increase in the nitrogen rate to 60 kg per 1 ha, seedlings died completely. When applying fertilizer in the same doses 5-6 cm away from the row of seeds and in the row with seeds, but 6 days after their sowing, no negative effect on the germination energy and field germination of seeds was observed. A soil layer of 5-6 cm well protected the seedlings from the poisonous effect of ammonia, and at the same time favorable conditions were created for the assimilation of it by plants.[ ...]
Nitrogen fertilizers. The main nitrogen fertilizer produced in the CMEA member countries is ammonium nitrate. In the GDR, only lime-ammonium nitrate is produced due to the explosive nature of ammonia. Ammonium sulphate is produced in relatively large quantities as a by-product in coke and caprolactam production in the USSR and the GDR. Recently, the production of urea (urea) as the most concentrated nitrogen fertilizer containing 45-46% N has increased dramatically. Other nitrogen fertilizers (sodium and calcium nitrate) are produced in small quantities. Of the liquid nitrogen fertilizers in the USSR, ammonia water is used in a relatively large volume; it is also used in the GDR and Poland. In the future, a transition is planned to the production of anhydrous ammonia containing 82% N.[ ...]
When coking coal, a significant amount of hydrogen sulfide is formed. Purification of gas from hydrogen sulfide with ammonia water makes it possible to obtain valuable preparations - ammonium sulfide and polysulfide, which are insectofungicides.[ ...]
According to the project, especially under the first option, the capital costs for the construction of an ammonia water plant are reduced by one quarter compared to the costs for the construction of a plant for the production of solid nitrogen fertilizers, since much less equipment, metal, pipes, cable products and other materials and simplifies plant layout. All equipment for the production of ammonia water from the conversion stage natural gas and ending with the ammonia synthesis shop, is located in one block. According to design developments, the cost of nitrogen contained in ammonia water is reduced by about 30-40% compared to nitrogen in solid fertilizers. Based on the VIUA data, the total cost of 1 ton of free nitrogen - soil could reach: in ammonia water 77.4%, in ammonium nitrate 100%, in carbamide 104% - Thus, and according to total costs, including the scope, ammonia water is more economical than solid fertilizers.[ ...]
In areas of insufficient moisture on chernozem soils, common forms of nitrogen fertilizers are ammonium sulphate and ammonium nitrate. Ols are used in small quantities in the main application together with phosphate fertilizers for winter wheat sown on non-fallow predecessors, corn and other tilled crops. Experiments carried out on chernozems have established a positive effect on the corn yield of new forms of nitrogen fertilizers - ammonia water and a more concentrated form - synthetic urea.[ ...]
The use of forms of nitrogen fertilizers for grain crops in the conditions of Bashkiria. The effectiveness of some forms of nitrogen fertilizers and methods of using ammonia water for corn on chernozems.[ ...]
The effect of liquid nitrogen fertilizers on the yields and quality of vegetable crops - table beets, tomatoes, early and late cabbages was also studied. All experiments confirmed that the use of ammonia water significantly increases the yield of vegetables. Similar results were obtained in experiments with grain crops (spring wheat, oats, barley). At the same time, liming the soil improved the effect of fertilizers.[ ...]
Studies have shown that urea is good fertilizer, especially when feeding winter rye and sugar beets. Extensive production tests have shown the benefits of using ammonia water as a fertilizer. At the same time, the urgent need to organize the transportation and introduction of ammonia water without loss of nitrogen became clear. To do this, it is necessary to organize the delivery and application of ammonia water to the collective farms and state farms by the combine in special emergency return tanks, as is done in the USA and other countries. It is also necessary to study the conditions for a more efficient use of ammonia water.[ ...]
Despite the fact that the post: wheat fell under heavy rain twice with hail? .: and the total yield was small (11.4 d / ha), the effect of fertilizers was clearly manifested. The yield increase from urea amounted to 2.2 centners, from ammonium sulfate - 4.5 centners, from ammonia water - 0.8 centners per hectare.[ ...]
Sodo-alkaline solution is fed into demineralized oil to bind thermally unstable calcium and magnesium chlorides. Neutralize organic acids and hydrogen chloride. Not most refineries practice the additional supply of ammonia water to the slurry lines of atmospheric columns to maintain the pH of the drainage water at a level of 8-9.[ ...]
In the regions of Eastern Siberia and the Far East, podzolic soils are most common, on which, as a rule, the use of nitrogen, phosphorus and potash fertilizers is very efficient. In extensive production tests on the use of ammonia water, carried out in 1957 in the Irkutsk region on areas of several thousand hectares, the increase in wheat grain yield from average doses of nitrogen in ammonia water was usually expressed by a value close to 10 c/ha.[ ... ]
Mineral fertilizers obtained from minerals as a result of mechanical or chemical processing, as well as synthetically. Mineral fertilizers according to the nature of their content nutrients are divided into five types: 1) nitrogen, 2) phosphorus, 3) potash, 4) lime and 5) microfertilizers. Of the nitrogen fertilizers currently the most common are: ammonium sulfate, ammonium nitrate, ammonium water and urea; from phosphorus: phosphate rock and superphosphate; from potassium; potassium chloride, sylvinite; from calcareous: ground limestone, meadow tuff, marl, defecation mud, gypsum; from microfertilizers: manganese, boric, copper and molybdenum.[ ...]
Purified flue gases pass through the stage of concentration by means of electrocyclic gas cleaning. After concentration, the volume of gases is 33.5 thousand m/h with a nitrogen oxide content of 21.4 g/m3 and a temperature of 29°C. Selective catalytic reduction with ammonia is used to purify these gases. When preparing technical proposals, two options were considered: 1 - using evaporated ammonia water as a reducing agent; 2 - gaseous ammonia.[ ...]
At the same time, the regeneration of solutions is complicated not only because of the lower ammonia pressure over the solutions, which can be ignored, since at 95-105 ° C both cyanides, bicarbonate, and ammonium sulfide and hydrosulfide are completely hydrolyzed. The complication is due to the fact that under these conditions, during the desorption of ammonia, the absorbed carbon dioxide, hydrogen cyanide, and other acid gases are inevitably desorbed. Therefore, it is impossible to obtain conditioned ammonia water or ammonia without additional purification of the solution or vapors.[ ...]
The method of sequential treatment of coal with sulfuric acid and ammonia is a universal method for the disposal of waste acids, both concentrated and diluted. It consists in the following: Sulfuric acid is processed) crushed tertiary brown coal, briquette dust or coal waste in ratios of 1: 1 or in other ratios. The resulting acidic mixture (products of coal sulfonation and excess sulfuric acid) is neutralized with gaseous ammonia, ammonia water or exhaust gases of nitrogen fertilizer industries containing ammonia. The obtained loose bulk mass can be used as a complex organic-ammonia fertilizer.[ ...]
When absorbing ammonia from gases containing carbon dioxide, it should be borne in mind that the absorption rate of the latter is limited by diffusion in the liquid phase. Therefore, it is more expedient to use devices for absorption in which a short contact time and intensive mixing of the gas phase (turbulent diffusion) are possible. In this case, the degree of absorption of both components of the gas mixture decreases, but the completeness of absorption of carbon dioxide turns out to be especially small. A short contact time is also used in the selective absorption of hydrogen sulfide by ammonia water from coke oven gas containing large quantities C02 .[ ...]
Ammonia production is a complex set of units, workshops, departments, apparatuses, interconnected by a technological chain. At various technological stages of ammonia production, from the synthesis column to the loading of liquid ammonia into railway tanks for shipment to the consumer, its emissions into the atmosphere are possible. The most significant emissions of ammonia into the atmosphere occur during the purging of inert gases and with tank gases formed when various containers are filled with ammonia. In addition, ammonia can be released into the atmosphere through various leaks in equipment, as well as when filling railway tanks. One of the possible solutions to the issue of protecting the air basin from ammonia emissions is the centralized collection and utilization of gas emissions with the production of ammonia water as a commercial product.
There is a chemistry class in every school, every university and most educational institutions. Aqueous ammonia is one of the substances that need to equip such an office. Let's take a closer look at what it is.
Binary compound of nitrogen and hydrogen, chemical formula which looks like this: NH3, is the most important of hydrogen compounds nitrogen is called ammonia. In total, there are several known compounds of hydrogen with nitrogen.
Aqueous ammonia is a clear liquid, which is nothing more than a solution of ammonia in water, which has a very pungent smell of ammonia. Another name for the solution, which can sometimes have a yellowish tinge, is ammonia water.
Ammonia technical liquid;
Water technical ammonia;
Water CHDA.
Chemical properties of ammonia
During chemical reactions in many cases, due to the presence of an indivisible electron pair, ammonia acts as a complexing agent. In another way, this is called the Bronsted base. Ammonia belongs to a number of reactive compounds. Due to the presence of the same unshared electron pair (at the nitrogen atom N), addition reactions are especially characteristic for ammonia. They are also easy to implement.
Getting aqueous ammonia
The German physicist discovered the physical and chemical basis of the method for obtaining ammonia in industry. This method is named after him - the Haber process. This industrial method for producing ammonia is based on the direct reaction of the interaction of two chemical elements: hydrogen and nitrogen. The formula for obtaining this compound at high temperature and pressure, using a catalyst, is as follows:
In order for the process of obtaining ammonia according to the formula to be successful, the following conditions must be met:
Temperature - 500 degrees Celsius;
Pressure - 350 atmospheres;
When using a catalyst, the ammonia yield is 3%.
During the reaction, heat is released and the volume decreases. In industrial conditions, the principle of circulation is more often used, when ammonia is removed, or withdrawn, by cooling. The nitrogen and hydrogen remaining outside the reaction are sent back (to the synthesis column). This process is more economical than the similar process, achieving a higher yield using high pressure.
Application
Ammonia water is very widely used in the chemical industry, being one of the important products. 100 million tons of ammonia are produced annually in the world. Ammonia water is used for the production of fertilizers based on nitrogen: ammonium nitrate and sulfate, urea. It also produces nitric acid, soda ash and polymers. It is used in the production of dyes, manganese, ferroalloys, and other electrolytes. Among other nitrogen fertilizers - ammonium nitrate, ammophoska, urea. Ammonia water is also used to produce some explosives and other chemical products, in the pharmaceutical and metallurgical industries.
Being a weak base when interacting with acids, ammonia water has a neutralizing effect on them. Aqueous ammonia is widely used for refrigeration technology and equipment. It is a refrigerant (R 717) and can also be used as a solvent.
Ammonia (10% solution of ammonia water) has found wide application in the medical field.
Ammonia water based serves in medicine as a reagent for analysis. It is used both in analytical chemistry and in chemical plants to obtain pure chemical products.
Ammonia water has found a fairly wide application in agriculture as a fertilizer and for the ammoniation of feed in animal husbandry.
Storage and transportation
It is better to transport ammonia water in glass or steel containers. It can also be stored in it for easy transportation. Liquid ammonia is transported in special chemical tanks designed for road freight and rail transport, in steel cylinders and tankers, as well as by moving through the pipeline.
The temperature of liquid ammonia, cooled and ready for transportation, should not exceed - 31.5 degrees Celsius. It is measured on the flange that connects the pipeline of the tanker and the loading line.
Aqueous ammonia is transported in sealed containers by rail, road and water transport in accordance with the rules for the transportation of high-risk goods in force on a particular type of vehicle. Tanks should be filled to 95%, no more. Hatches must be sealed and must be sealed.
Ammonia water is divided into two types. Grade A product is transported in ammonia carriers and railway tanks. Grade B is transported in tanks with bottom drains and in ammonia carriers.
Precautionary measures
At normal temperatures and atmospheric pressure ammonia has a gaseous state and is a combustible gas. Ammonia gas can spontaneously ignite at a temperature of 650 degrees Celsius, the minimum ignition energy is approximately 680 mJ.
A mixture of ammonia and air in a ratio of 15-28 to 100 is explosive, and liquid ammonia belongs to the class of slow-burning substances.
To extinguish fires of liquid or gaseous ammonia, automatic fire extinguishing installations filled with water, non-combustible gas or foam are used.
Ammonia is a toxic compound with a maximum allowable concentration active substance(MAC) in the air of working areas of industrial premises 20 mg/m 3 .
Gaseous ammonia can cause lacrimation and suffocation in a person due to acute irritation of the mucous membranes of the throat, nasal and oral cavities, and eyes.
If a jet of gas or a drop of liquid ammonia gets on the skin, severe burns may result. In this case, the affected area should be immediately washed with a flowing jet. cold water and apply lotions (containing 3-5% citric or acetic acid).
If the victim inhaled air with a high content of ammonia, he needs to urgently go to fresh air so that the respiratory organs are cleared.
If liquid ammonia gets into the mucous membrane of the eyes, rinse the eyes quickly with plenty of water.
Precautions when handling liquid ammonia
If your work involves interaction with this substance in any form, you must follow the rules:
Use individual funds for protection (gas mask, mask, protective apron or suit;
Hands should be protected from frostbite: wear insulated rubber gloves;
To protect the feet in winter, rubber boots or felt boots with rubber soles are issued at the factory, and rubber boots or other rubberized shoes are worn in summer.


