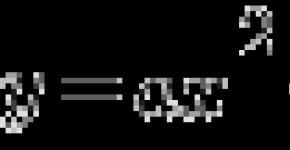Water Structure: New Experimental Data. Water structure
In the water molecule, the main acting person is an oxygen atom.
Since hydrogen atoms from each other are noticeably repelled, the angle between chemical bonds (lines connected by the nuclei of atoms) hydrogen - oxygen not straight (90 °), and a little more - 104.5 °.
Chemical bonds in the water molecule - polar, as oxygen pulls against themselves negatively charged electrons, and hydrogen is positively charged electrons. As a result, an overnight negative charge is accumulated near the oxygen atom, and the hydrogen atoms are positive.
Therefore, the whole water molecule is a dipole, that is, a molecule with two variemety poles. The dipole structure of the water molecule largely determines its unusual properties.

Water molecule is a diamagnet.
If you connect with straight lines of epicenters of positive and negative charges, it turns out volumetric geometric figure - Tetrahedron. This is the structure of the water molecule itself.
With a change in the state of the water molecule, the length of the parties and the angle between them vary in the tetrahedra.
For example, if the water molecule is in a vapor state, the angle formed by it by the sides is 104 ° 27. "In the aqueous state angle is 105 ° 03." And in the ice state an angle is 109.5 °.

Geometry and dimensions of water molecule for various states
A - for a vapor
B - for the lowest oscillatory level
B - for a level close to the formation of ice crystal when the geometry of water molecule corresponds to the geometry of two Egyptian triangles with the aspect ratio of 3: 4: 5
G - for ice condition.
If these angles are divided in half, then we get angles:
104 ° 27 ": 2 \u003d 52 ° 13",
105 ° 03 ": 2 \u003d 52 ° 31",
106 ° 16 ": 2 \u003d 53 ° 08",
109.5 °: 2 \u003d 54 ° 32 ".
So, among the geometric patterns, the water and ice molecule is the famous Egyptian triangle, which is based on the basis of which the ratios of the gold proportion are laid - the lengths of the sides are as 3: 4: 5 with an angle of 53 ° 08.
The water molecule acquires the structure of the golden proportion on the way when water goes into ice, and vice versa when the ice melts. Obviously, for this state and melt water is valued, when its structure in the construction has the proportions of the golden cross section.
Now it becomes clear that the famous Egyptian triangle with the aspect ratio of 3: 4: 5 "taken" from one of the states of the water molecule. The geometry of the water molecule is formed by two Egyptian rectangular triangleshaving a total nut equal to 3.
Water molecule based on a gold proportion is the physical manifestation of Divine Nature, which is involved in creating life. That is why the harmony is laid in the earthly nature, which is inherent in all space.
And therefore, the ancient Egyptians deified numbers 3, 4, 5, and the triangle himself was considered sacred and tried to lay its properties, his harmony in any design, houses, pyramids and even in the marking of the fields. By the way, the Ukrainian huts were also constructed using the ratio of the gold proportion.

In the space of water molecule, there is some volume, and coated with an electronic shell in the form of a veil. If you present the form of a hypothetical model of a molecule in a plane, then it looks like a butterfly wings, on the X-shaped chromosome, in which the livestock life program is recorded. And this is an indicative fact that water itself is mandatory element Total alive.
If you represent the form of a hypothetical model of water molecule in volume, then it transmits the shape of a triangular pyramid, which has 4 faces, and each face has 3 ribs. In geometry, the triangular pyramid is called tetrahedrome. This structure is characteristic of crystals.
Thus, the water molecule forms a solid corner structure, which it retains even when it is in a vapor state, on the verge of transition to ice, and when turns into ice.
If the "skeleton" of the water molecules are so stable, then its energy "pyramid" - the tetrahedron is also unshakable.
Such structural properties of water molecule in different conditions Explained by durable bonds between two hydrogen atoms and one oxygen atom. This connection is about 25 times stronger than the relationship between adjacent water molecules. Therefore, it is easier to separate one water molecule from another, for example, when heated than to destroy the water molecule itself.
Due to the orientational, induction, dispersion interactions (Van der Waals forces) and hydrogen bonds between hydrogen atoms and oxygen, the adjacent molecules of water molecule are capable of forming both random associates, i.e. There are no ordered structures and clusters - associates having a certain structure.
According to statistical data, ordinary water There is random associates - 60% (destructured water) and clusters - 40% (structured water).
As a result of research conducted by the Russian scientist S. V. Zhenin, stable long-lived clusters of water were discovered.
Zenin found that water molecules originally form a dodecahedron. Four dodecahedra connecting, forms the main structural element of water - a cluster consisting of 57 water molecules.

The dodecahedra cluster has common areas, and their centers form the right tetrahedron. This is a volume compound of water molecules, including hexamers, which has positive and negative poles.
Hydrogen bridges allow water molecules to combine the most different ways. Due to this, in the water there is an infinite variety of clusters.
Clusters can interact with each other due to free hydrogen bonds, which leads to the appearance of second-order structures in the form of hexagons. They consist of 912 water molecules that are practically not able to interact. The existence of such a structure is very large.

This structure, similar to a small acute crystalline of ice from 6 rhombic faces, S.V. Zenin called the "main structural element of water". Numerous experiments were confirmed; in water - the myriad of such crystals.
These crystals of ice almost do not interact with each other, therefore do not form more complex stable structures and easily slide with their faces relative to each other, creating fluidity. In this sense, water resembles a supercooled solution that cannot crystallize.
The composition of water can be found out using the decomposition reaction electric shock. Two volumes of hydrogen for one oxygen volume (gas volume is proportional to the amount of substance):
2H 2 O \u003d 2H 2 + O 2
Water consists of molecules. Each molecule contains two hydrogen atoms connected by covalent bonds with one oxygen atom. The angle between connections is about 105 °:
O - H.
H.
Since oxygen is a more electronegative element (strong oxidizing agent), a total electron pair of covalent bonds shifts to an oxygen atom, a partial negative charge Δ- is formed on hydrogen atoms - partial positive Δ +. Neighboring molecules are attracted to each other with opposite charges - this causes a relatively high boiling point of water.
Water room temperature - Colorless transparent liquid. Melting point 0º C, boiling point at atmospheric pressure - 100 ° C. Clean water does not conduct an electric current.
An interesting feature of water is that it has the highest density of 1 g / cm 3 at a temperature of about 4 ° C. With a further decrease in temperature, the water density decreases. Therefore, with the onset of winter, the upper freezing layers of water becomes easier and do not dive down. The ice is formed on the surface. Provisions of the reservoir to the bottom usually does not occur (moreover, ice also has a density less water And swims on the surface).
Chemical properties:
To main pollutants natural water relate wastewater industrial enterprisescontaining mercury compounds, arsenic and other toxic elements. Streams of livestock complexes, cities may contain waste causing the rapid development of bacteria. The lack of danger to natural reservoirs is incorrect storage (not providing protection against precipitation) or the use of fertilizers and eradicates washped in reservoirs. Transport, especially water, pollutes water bodies with petroleum products and household garbageemitted unscrupulous people directly into the water.
To protect the waters, it is necessary to introduce closed water supply of industrial enterprises, comprehensive processing of raw materials and waste, construction claimed facilities, Environmental education of the population.
* For water electrolysis, salting solutions are used
2. Experience. Recognition of carbonic acid salt among the three proposed salts.
The qualitative reaction to carbonates is the interaction with acids, accompanied by stormy separation of carbon dioxide:
Caco 3 + 2HCl \u003d CaCl 2 + H 2 O + CO 2
or, in ion form:
CO 3 2- + 2H + \u003d H 2 O + CO 2
To prove that it is precisely carbon (IV) oxide (IV), you can, passing it through a solution of lime water, which causes her clouds:
CO 2 + Ca (OH) 2 \u003d Caco 3 ↓ + H 2 O
To recognize the salt of coalic acid, add a little acid to all three test tubes (so that it does not pour over the edge when "boiling"). Where the colorless gas without smelling will be distinguished, carbonate is located there.
Water can be in three aggregate states - gaseous, liquid and solid. In each of these states, the structure of water is not the same. Depending on the composition of substances in it, water acquires new properties. The solid state of water also happens at least two types: crystalline - ice and non-crystalline - glassy, \u200b\u200bamorphous (vitrification status). With instant freezing with, for example, liquid nitrogen molecules do not have time to be built into the crystal lattice, and water acquires a solid glass-like state. It is this property of water that allows you to freeze living organisms without damage, such as single-cell algae, MCI sheet leaflets, consisting of two cell layers. Freezing with the formation of crystalline water leads to damage to cells.
For the crystal state of water, a large variety of forms is characterized. It has long been noticed that the crystalline water structures resemble radolaries, fern leaves, cysts. On this occasion, A. A. Lyubshchev suggested that the laws of crystallization are similar to the laws of the formation of living structures.
Physical properties of water. Water is the most abnormal substance, although the measure of density and volume for other substances is adopted.
Density. All substances increase the volume when heated, reducing the density. However, at a pressure of 0.1013 MPa (1 atm.) In the water in the range from 0 to 4 0 s, with an increase in temperature, the volume decreases and the maximum density is observed (at this temperature 1 cm 3 of water we have a lot of 1g). When freezing, the volume of water increases sharply by 11%, and when ice melted at 0 ° C, the same sharply decreases. With increasing pressure, the water freezing temperature drops after every 13.17 MPa (130 atm.) To 1 0 C. Therefore, at high depths at minus temperatures, water in the ocean does not freeze. With increasing temperature of up to 100 0 with the density of liquid water, it drops by 4% (at 4 ° C, its density is equal to 1).
Boiling and freezing points (melting). With a pressure of 0.1013 MPa (1 atm.) The freezing and boiling points of water are at 0 ° C and 100 ° C, which sharply distinguishes H20 from hydrogen compounds with elements of the group VI periodic system Mendeleeva. In the row of H2T, H2SE, H2S, etc. With the increase in the relative molecular weight of the boiling point and the freezing of these substances increase. In compliance with this rule, water would have to have a freezing point between - 90 and - 120 ° C, and boiling - between 75 and 100 ° C. The boiling point of water increases with an increase in pressure, and the freezing temperature (melting) is dropped (Appendix 1).
Heat melting. The hidden heat of melting ice is very high - about 335 J / g (for iron - 25, for sulfur - 40). This property is expressed, for example, in the fact that ice normal pressure May have a temperature from - 1 to - 7 ° C. The hidden heat of water vaporization (2.3 kJ / g) is almost 7 times higher than the hidden heat of melting.
Heat capacity. The magnitude of water heat capacity (i.e., the amount of heat required to increase the temperature by 1 ° C) is 5-30 times higher than that of other substances. Only hydrogen and ammonia possess greater heat capacity. In addition, only liquid water and mercury, the specific heat capacity with an increase in temperature from 0 to 35 ° C drops (then starts to increase). The specific heat capacity of water at 16 ° C is conditionally adopted per unit, serving the standard for other substances. Since the heat capacity of the sand is 5 times less than that of liquid water, then with the same heating the sun, the water in the reservoir is heated 5 times weaker than sand on the shore, but in the same time it retains the warmth. High water heat capacity protects plants from a sharp increase in temperature when high temperatures The air, and the high heat of the vaporization participates in thermoregulation in plants.
High melting and boiling temperatures, high heat capacity indicate a strong attraction between adjacent molecules, as a result of which liquid water It has a large inner clutch.
Water as a solvent. The polarity of water molecule determines its property to dissolve substances better than other liquids. The dissolution of the crystals of inorganic salts is carried out due to the hydration of ions included in their composition. Well dissolve in water organic substances, with carboxyl, hydroxyl. Carbonyl and with other groups that water forms hydrogen bonds. (Appendix 1)
The water in the plant is both in free and in the associated state (Appendix 2). Free water - mobile, it has almost all physico- chemical properties Clean water, well penetrates through cell membranes. There are special membrane proteins that form inside the membrane channels permeable for water (aquaporins). Free water enters various biochemical reactions, evaporates in the process of transpiration, freezes at low temperatures.
Related water - has changed physical properties mainly as a result of interaction with non-aqueous components. Conditionally adopted under the tied water that does not freeze with a decrease in temperature to - 10 ° C.
Related water in plants happens:
1) Osmotically - associated
2) colloidal-related
3) capillary-related
Osmotically associated water is associated with ions or low molecular weight substances. Water hydrates dissolved substances - ions, molecules. Water electrostatically binds and forms a monomolecular layer of primary hydration. Vacuolar juice contains sugar, organic acids and their salts, inorganic cations and anions. These substances hold the water osmotically.
Colloid-bound water - includes water, which is inside a colloidal system and water, which is on the surface of colloids and between them, as well as immobilized water. Immobilization is a mechanical seizure of water in conformational changes of macromolecules or their complexes, while water turns out to be concluded in closed space macromolecules. A significant amount of colloid-bound water is on the surface of the cell wall fibrils, as well as in the cytoplasm biocolloids and the matrix of the membrane cell structures.
Water, hydrating colloidal particles (primarily proteins), is called colloid-bound, and solutes ( mineral salts, sugar, organic acids, etc.) - osmotically connected. Some researchers believe that all water in the cell is to one degree or another associated. Physiologists are conventionally understood under the tied water that does not freeze with a decrease in temperature up to-10 ° C. It is important to note that any binding of water molecules (adding solutes, hydrophobic interactions, etc.) reduces their energy. This is the basis of a decrease in the water potential of the cell compared to clean water.
The water content in various organs of plants varies in fairly wide limits. It varies depending on the conditions external environment, age and plant species. Thus, the water content in the leaves of salad is 93-95%, corn - 75-77%. The amount of water is different in different organs of plants: in the leaves of sunflower water contains 80-83%, in the stems - 87-89%, in the roots - 73-75%. The water content equal to 6-11% is characteristic mainly for air-dry seeds, in which the processes of vital activity are inhibited. Water is contained in alive cells, in the dead elements of xylems and in interclausers. In interclauders, water is in a vapor state. The main evaporating organs of the plant are the leaves. In this regard, it is natural that the greatest amount of water fills the interclatures of the leaves. In liquid state, water is in different parts Cells: cell shell, vacuole, protoplasm. The vacuole is the richest part of the cage, where it is 98% reaches its content. With the greatest hoe, the water content in the protoplasm is 95%. The smallest content Waters are characteristic of cell membranes. Quantitative determination of water content in cellular shells is difficult; Apparently, it varies from 30 to 50%.
Water form B. different parts Vegetable cells are also different. In vacuolar cellular juice, water predominates with relatively low molecular weight compounds (osmotic-related) and free water. In the shell of the plant cell, water is mainly associated with high-polymer compounds (cellulose, hemicellulose, pectin substances), i.e. colloid-bound water. In the cytoplasma itself there is a free, colloid and osmotically connected. Water, located at a distance of up to 1 nm from the surface of the protein molecule, is firmly connected and does not have the correct hexagonal structure (colloid-bound water). In addition, in the protoplasm there is a certain number of ions, and, consequently, part of the water is osmotically connected.
The physiological value of free and bound water is different. Most researchers believe that the intensity of physiological processes, including growth rates, is primarily dependent on the content of free water. There is a direct correlation between the connected water content and the resistance of plants against adverse external conditions. Indicated physiological correlations are not always observed.
Candidate of Chemical Sciences Alexander Smirnov, Professor Miera.
Water given mysterious power
Being juice of life on earth.
Leonardo da Vinci
Fig. 1. Water structure at a temperature of 20 aboutC, horizontal size - 400 μm. White spots are emulsions.
Fig. 2. Structure aqueous solutions at 20. aboutC: A - distilled water; B - degasive mineral water Borjomi; IN - alcohol tincture 70%.
Fig. 3. Emulons in bidistillated water at temperatures 4 aboutC (a), 20 aboutC (b), 80 aboutC (B). Sizes of shots 1.5 × 1.5 mm.
Fig. 4. Change the amplitude of the signals of acoustic emission and water temperature during the ice melting process.
Fig. 5. Relative temperature change when water heating.
Details for curious. Scheme of experience. Per a short time From a cup with a positive electrode (anode) through the "bridge" flowed 0.5 grams of water.
"Steaming water bridge" about 3 centimeters long.
The electrified glass wand distorts the shape of the "bridge" and breaks it on the trickle.
So might look emuls that form the thread-shaped structure of the "bridge".
The water is customary to be considered and as a practically neutral solvent in which biochemical reactions flow, and as a substance spread through the body of living organisms. At the same time, water is an indispensable participant of all physicochemical processes and, by virtue of their vast importance, the most studied substance. The study of the properties of water has repeatedly resulted in unexpected results. It would seem that some surprises can be in themselves a simple reaction of hydrogen oxidation 2H 2 + O 2 → 2H 2 O? But the work of Academician N. N. Semenova showed that this reaction is branched, chain. It was more than seventy years ago, and about chain reaction Uranium divisions have not yet known. Water in a glass, river or lake is not just huge amounts of individual molecules, but their associations, supramolecular structures - clusters. To describe the water structure, a number of models are proposed that only some of its properties explain more or less, and in relation to others contradict the experiment.
theoretically, clusters are usually calculated only for several hundred molecules or for a layer near the interfacial boundary. However, a number of experimental facts testifies that there may be gigantic, molecular magnitude, structure (work-corresponding member of the Russian Academy of Sciences E. E. Fesenko).
In the thoroughly purified double distilled water and some solutions, we were able to detect the structural formations of the five fractions with dimensions from 1 to 100 microns using laser interferometry. Experiments made it possible to establish that each solution has its own inherent in the structure only to it (Fig. 1, 2).
Outmoolecular complexes are formed by hundreds of thousands of water molecules grouped around hydrogen ions and hydroxyl in the form of ion pairs. For these supramolecular complexes, we offer the name "Emulons" to emphasize their similarity with particles forming the emulsion. Complexes consist of separate fractions with dimensions from 1 to 100 microns, and fractions having dimensions of 30, 70 and 100 μm, much more than the others.
The content of individual fractions of emulsion depends on the concentration of hydrogen ions, temperature, the concentration of the solution and the prehistory of the sample (Fig. 3). In bidistillated water, with 4 o, the complexes are tightly packed and form a texture resembling parquet. As is known, water at this temperature has the maximum density. With increasing temperature up to 20 ° C in the structure of water, significant changes occur: the number of free emuls becomes the greatest. With further heating, they gradually destroy, the number decreases, and this process is mainly ends at 75 ° C, when the speed of sound in water reaches a maximum.
Due to the long-range effect of electrostatic forces, emulsions in water form a rather stable ultra-dryer, which, however, reacts sensitively to electromagnetic, acoustic, thermal and other external influences.
The discovered supramolecular complexes have consistently include all previously obtained information about the organization of water in nano-beams and allow you to explain many experimental facts that did not have a slim, logical justification. These include, for example, the formation of a "soaring water bridge" described in a number of works.
The essence of the experiment lies in the fact that if you put two small chemical glasses with water nearby, lower the platinum electrodes under the constant voltage of 15-30 kV, then the water jumper is formed with a diameter of 3 mm and up to 25 mm long. The "bridge" steer for a long time, has a layered structure, and water transfer from the anode to the cathode. This phenomenon and all its properties are a consequence of emuls in water, which seems to have a dipole moment. One more property can be predicted: at a water temperature above 75 o, the bridge will not arise.
Easy to explain the abnormal properties of melt water. As noted in the literature, many properties of melt water - density, viscosity, electrical conductivity, refractive index that dissolves the ability and others - differ from the equilibrium parameters. Communication of these effects to the removal of deuterium from the water as a result of a phase transition (melting point " heavy ice"D 2 O 3.82 O C) is untenable, since the concentration of deuterium is extremely insignificant - one atom of deuterium by 5-7 thousand hydrogen atoms.
The study of ice melting by the method of acoustic emission made it possible for the first time that, after complete melting of ice, melt water, which is in a metastable state, becomes a source of acoustic pulses, which serves as experimental confirmation of the formation of ollocularities in water (Fig. 4).
Experiments show that melt water for almost 17 hours can be in an active metastable state (after melting ice, its microcrystalline is saved only the fractions of the second and do not determine the properties of melt water). This mysterious phenomenon is explained by the fact that when the destruction of hexagonal crystal lattice Ice varies dramatically the structure of the substance. Ice crystals are destroyed faster than it is rebuilt into the steady equilibrium condition of water formed from it.
The uniqueness of the phase transition of the LodaVode is that in melt water, the concentration of hydrogen ions H + and hydroxyl OH - a short time remains non-equilibrium, which it was in ice, that is, a thousand times less than in ordinary water. After some time, the concentration of H + and OH ions - in water takes its equilibrium value. Since hydrogen and hydroxyl ions play a crucial role in the formation of supramolecular water complexes (emuls), water remains in a metastable state for a while. The reaction of its dissociation H 2 O → H + + OH - requires significant energy costs and proceeds very slowly. The rate constant of this reaction is only 2.5 ∙ 10 -5 C -1 at 20 ° C. Therefore, the time of return of water to the equilibrium state theoretically should be 10-17 hours, which is observed in practice. Research the dynamics of changes in the concentration of hydrogen ions in thawed water in time confirm this. The unusual properties of melt water serve as a conversation about the "memory" of water. But under the "memory" of water should be understood by the dependence of its properties from the background and nothing more. Can different ways - freezing, heating, boiling, treatment with ultrasound, exposure to various fields, etc. - translate water into a metastable state, but it will be unstable, which will not retain its properties. We discovered the presence of only one fraction of supramolecular formations with dimensions of 1-3 microns in the optical method. It is possible that the reduced viscosity and a more rare spatial mesh from emulsion in melt water increases the dissolving ability and diffusion rate.
The reality of the existence of emulsion confirms classic method thermal analysis (Fig. 5). The graph is observed clearly pronounced peaks, testifying to structural restructuring in water. The most significant corresponds to 36 o C - the temperature of the minimum heat capacity, 63 o C - the temperature of the minimum compressibility, and is especially characterized by peak at 75 o C - the temperature of the maximum sound speed in the water. They can be interpreted as peculiar phase transitions associated with the destruction of emulsion. This allows us to conclude: liquid water is a very peculiar dispersed system, which includes at least five structural formations with various properties. Each structure exists in a certain characteristic of her temperature range. The temperature exceeding the threshold level is critical for this structure leads to its decay.
Literature
Zatsepina G. L. Physical properties and water structure. - M.: Publishing House of Moscow University. - 1998. - 185 p.
Kuznetsov D. M., Gaponov V. L., Smirnov A. N. On the possibility of studying the kinetics of phase transitions in a liquid medium by the method of acoustic emission // Engineering physics, 2008, No. 1, p. 16-20.
Kuznetsov D. M., Smirnov A.N., Syroeshkin A.V. Acoustic emission with phase transformations in aquatic environment // Russian Chemical Journal - M.: Ros. Chem. about them. D. I. Mendeleeva, 2008, vol. 52, No. 1, p. 114-121.
Smirnov A. N. Water Structure: New Experimental Data. // Science and Technology in Industry, 2010, No. 4, p. 41-45.
Smirnov A. N. Acoustic emission in the flow of a chemical reaction and physicochemical processes // Russian Chemical Journal. - M.: Ros. Chem. about them. D. I. Mendeleeva, 2001, vol. 45, p. 29-34.
Smirnov A.N., Syroyshkin A.V. Supratmolecular water complexes // Russian Chemical Journal. - M.: Ros. Chem. about them. D. I. Mendeleeva, 2004, vol. 48, No. 2, p. 125-135.
Details for curious
How does the "bridge" arise
The formation of the "Water Bridge" is described in the works of the Netherlands Physics of Elmara Fuchs with colleagues.
In two standing nearby Small water tanks immerse platinum electrodes and are fed by a constant voltage of 15-20 kV. In photographs, it is clearly seen that at first in anodic glass, and then in the cathode on the surface of the water there are elevations, which merge, forming a water jumper with a diameter of 2-4 mm between the capacitors. After that, the glasses can be pulled away from another 20-25 mm. The jumper exists for quite a long time, forming a "soaring water bridge". Along the "bridge" flows water. The ends of the "bridge" are varieved, so water in the capacles acquires various values pH: 9 and 4. "Bridge" consists of thin pips; When applying to it, a charged glass wand is split into several sleeves. The high technique of the experiment allowed to register the movement of spherical formations over the surface of the "Water Bridge".
Water is the most common connection in live systems. But the water content varies widely: from 10% (the enamel of the teeth), 20% (bone tissue), up to 85% (human brain), in dry seeds 10-12%, in jellyfish 95-98%, i.e. . The whole body essentially consists of water. Loss of 20% of water leads to cell death or anabyosis.
Water properties are unique, i.e. No other connection has them. This is due to the structure of its molecules: one oxygen atom is associated with a durable covalent bond with two hydrogen atoms, i.e. H 2 O is a very simple connection. Hydrogen atoms are attached to oxygen at an angle of 104.5 0.
Fig.1. The structure of water molecule.
Features physical properties Waters are associated with the structure of its molecule and the peculiarities of intermolecular interactions. The distribution of the electron density in the water molecule is such (Fig. 1, b, c), which is created 4 poles of charges: 2 positive, associated hydrogen atoms, and 2 negative associated with electron electron clouds of an oxygen atom. The indicated 4 poles of charges are located in the tetrahedra vertices (Fig. 1, d). Due to this, the dipole water molecule, and the four poles of charges allow each molecule to form four hydrogen bonds with adjacent (the same) molecules. As a result, clusters are formed (with instant freezing, they look like beautiful snowflakes, Fig.2.).


Fig.2. Water cluster formation.
Clusters form workers "Water structure". The hydrogen bonds are weak, 15-20 times weaker covalent. Therefore, some connections are easy to rush, others arise. As a result, the molecules are very mobile. Any external changes (temperatures, pressure, etc.) change this workforce. Thus, water has high sensitivity and memory.
Water molecules can be connected to molecules carrying electronic charge, as a result, hydrates are formed. If the force of attraction between water molecules is less than the attraction of water to the molecules of the substance - the substance dissolves.
Properties and water functions.
1. Ties in a single system all living and non-living nature on the planet. The water is movable, changeable, but not changing chemical composition molecules, and the structure of the cluster.
2. Water - universal solvent. Due to polarity, it does not have equal in it: more substances dissolves in water than in any other liquids. Substances in the cell come and are removed only in dissolved form.
3. In relation to water, the substance in the cell is divided into 2 groups:
a) hydrophobic (FOBOS - fear, horror): insoluble in water (fats, polysaccharides, etc.)
b) Hydrophilic (Fileon - love): soluble in water (mineral salts, acids, monosaccharides, etc.)
Due to this property of water (due to hydrophobic interactions) in the cell collected:
1) biological membranes,
2) Proteins and DNA take the shape of a spiral.
4. Water is characterized by high heat capacity (that is, to raise the temperature of the water and break the hydrogen bonds requires a lot of energy). So the boiling point of water 100 0 s, and alcohol 70 0 C.
5. High thermal conductivity. Due to this property in the cell and the body, thermal equilibrium is maintained.
6. Water itself as chemical compound Participates in many chemical reactions. For example, hydrolysis reactions go through water connection.
7. It is a source of 2 and H + with photosynthesis (photoliz of water).
8. Water is the main medium for vehicles of substances in the cell (diffusion) and the body (blood and lymph currents, the interstitial liquids containing nutrients, O 2 and CO 2, hormones, substances that include and turn off genes). This is a transport function.
9. Provides the volume and elasticity of the cell: the tour and osmotic pressure, retains the shape of cells and organisms (the hydraulic cooler at round and ring worms).
10. Wednesday for fertilization.
11. Wednesday for the lives of aquatic organisms.
12. Wednesday for the development of nuclei of animals (in amnion).
13. Participates in the formation of lubricating fluids in the joints, pleural cavity, a window-shaped bag.
14. Forms mucus providing the movement of substances by intestines, a wet medium on mucous membranes (sneezing, cough).
15. Participates in the formation of secrets (saliva, tears, bile, sperm and salt in the body).
16. Water is a limiting factor of life on our planet. Everywhere where there is water, there is a life where there is no water - there is no life there.