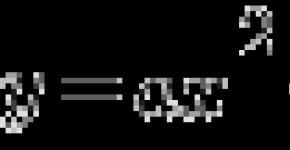Quantum dots are nanoscale sensors for medicine and biology. Quantum dots (Quantum Dot LED) - New Display Production Technology
COURSE WORK
under the discipline "Biomedical converters and sensory systems"
Quantum dots and biosensors based on them
Introduction 3.
Quantum dots. General. 5
Classification of quantum dots. 6.
Photoluminescent quantum dots. nine
Getting quantum dots. eleven
Bosensors using quantum dots. Prospects for their use in clinical diagnostics. 13
Conclusion. fifteen
Bibliography. sixteen
Introduction
Quantum dots (CT) are isolated nanoobjects whose properties differ significantly from the properties of the volume material of the same composition. Immediately it should be noted that quantum dots are more like a mathematical model than real objects. And this is due to the impossibility of the formation of full-resistant structures - small particles always interact with environmentalWhile in a liquid medium or solid matrix.
To sort out what quantum dots are, and understand them electronic structure, imagine an ancient Greek amphitheater. Now imagine that the scene unfascisives, and the audience ranks are filled with the public who came to watch the game of actors. So it turns out that the behavior of people in the theater is largely similar to the behavior of the electrons of the quantum point (CT). During the presentation, the actors move in the arena, without leaving the audience, and the audience themselves follow the action from their places and do not go down on the stage. Arena is the lower completed levels of the quantum point, and the audience ranks are excited electronic levels with higher energy. At the same time, as a viewer can be in any row of the hall, and the electron is able to occupy any energy level of the quantum point, but cannot be located between them. Buying tickets to the idea at the box office, everyone sought to get the most best places - As close as possible to the scene. Indeed, well, who wants to sit in the last row, from where the actor's face does not consider even in binoculars! Therefore, when the audience is searched before the view of the presentation, all the lower rows of the hall are filled, as well as in the stationary state of the CT, which has the lowest energy, the lower energy levels are fully occupied by electrons. However, during the presentation, someone from the audience may leave their place, for example, because the music on the stage plays too loudly or just a neighbor is nice caught, and reconcile to a free top row. This is how and in CT, the electron under the action of external influence is forced to switch to a higher energy level, not occupied by other electrons, leading to the formation of the excited state of the quantum point. Probably, you wonder what happens with the empty place at the energy level, where was the electron - the so-called hole before? It turns out that the electron electron is connected with her charge interactions and can go back at any time, as well as the viewer can always change and return to the place marked in his ticket. A pair of "electron-hole" is called "exciton" from english word. "Excited", which means "excited". Migration between energy levels CT, similar to the rise or descent of one of the audience, is accompanied by a change in the electron energy, which corresponds to the absorption or radiation of the light quanta (photon) when the electron is transition, respectively, to a higher or low level. The above-described behavior of electrons in a quantum point leads to a discrete energy spectrum uncharacteristic for macro objects, for which CT is often called artificial atoms in which the electron levels are discrete.
The strength (energy) of the connection holes and the electron determines the exciton radius, which is a characteristic value for each substance. If the particle size is less than the exciton radius, then the exciton is limited in the space by sizes, and the corresponding bond energy varies significantly compared with the volumetric substance (see "Quantum-based effect"). It is not difficult to guess that if the energy of the exciton changes, the energy of a photon emitted by the system changes during the transition of an excited electron to its original place. Thus, obtaining monodisperse colloidal solutions of nanoparticles of various sizes, you can control the transition energies in a wide range of optical spectrum.
Quantum dots. General.
The first quantum dots were nanoparticles of metals that were synthesized in ancient Egypt For coloring of various glasses (by the way, the Kremlin's ruby \u200b\u200bstars were obtained on the close technology), although more traditional and well-known CT are semiconductor GAN semiconductor particles and colloidal solutions of CDSE nanocrystals. At the moment, many ways to obtain quantum points are known, for example, they can be "cut" from thin layers of semiconductor "heterostructures" using "nanolithography", and it is possible to spontaneously form in the form of nano-dimensional inclusions of the structures of the semiconductor material of the same type in the matrix of the other. The method of "molecular-beam epitaxy" with a significant difference in the parameters of the elementary cell of the substrate and the sprayed layer can be achieved on the substrate of pyramidal quantum dots, for the study of the properties of which the Nobel Prize was awarded to the Academician J.I. Alferov. Controlling the conditions for the synthesis processes, theoretically, you can get quantum points of certain sizes with specified properties.
Quantum dots are available both in the form of cores and as a heterostructures of the type core-shell. Because of the small size, the CT has properties other than bulk semiconductors. The spatial limitation of the movement of the charge carriers leads to a quantum-dimensional effect expressing in the discrete structure of electronic levels, which is why CT is sometimes called "artificial atoms".
Depending on the size and chemical composition Quantum dots have photoluminescence in visible and neighboring infrared bands. Due to the high homogeneity in size (more than 95%), the proposed nanocrystals have narrow emission spectra (half width of the fluorescence peak of 20-30 nm), which ensures phenomenal color purity.
Quantum dots can be supplied in the form of solutions in non-polar organic solvents, such as hexane, toluene, chloroform, or in the form of dry powders.
CT is still a "young" object of research, but wide prospects for their use for the design of lasers and displays of a new generation are already quite obvious. The optical properties of the CT are used in the most unexpected areas of science that require the rebuilt fluorescent properties of the material, for example, in medical studies with their help it turns out to be able to "highlight" patients with tissue.
Classification of quantum dots.
Colloid synthesis of quantum dots represents wide opportunities Both in obtaining quantum points based on various semiconductor materials and quantum points with different geometry (shape). An important is the possibility of synthesizing quantum points composed of different semiconductors. Colloid quantum dots will be characterized by the composition, size, shape.
- Composition of quantum dots (semiconductor material)
First of all, quantum points are of practical interest as luminescent materials. The basic requirements for semiconductor materials on the basis of which quantum points are synthesized are the following. First of all, it is the styling nature of the zone spectrum - provides an effective luminescence, in the second, the small effective mass of charge carriers is the manifestation of quantum-dimensional effects in a fairly wide range of sizes (of course by the standards of nanocrystals). The following classes of semiconductor materials can be distinguished. Widget semiconductors (Zno, TiO2 oxides) - ultraviolet range. Medium semiconductors (A2B6, such as Cadmium Chalcogenides, A3B5) -vidable range.
Ranges of change of the effective width of the forbidden zone of quantum dots when
resizing from3 to 10 nm.
The figure shows the possibility of varying the effective width of the prohibited zone for the most common semiconductor materials in the form of nanocrystals with a size of 3-10 nm. From a practical point of view, important optical ranges are visible 400-750 nm, Middle IR 800-900 nm - blood transparency window, 1300-1550 Nm -Telecommunication range
- Form of quantum dot
In addition to the composition and size, a serious impact on the properties of quantum dots will be their form.
- Spherical (directly Quantum Dots) - most of the quantum dots. Currently have the largest practical application. The most simple in the manufacture.
- Elypsoidal(Nanorods) - nanocrystals, elongated along one direction.
Eleviption coefficient 2-10. These boundaries are conditional. From a practical point of view, this class of quantum points is used as sources of polarized radiation. With large elipethity coefficients\u003e 50, this type of nanocrystals is often called threads (nanowires).
- Nanocrystals with complex geometry (for example, TetRapods). A sufficient variety of forms - cubic, asterisks, etc., as well as branched structures can be synthesized. From a practical point of view, Tetrapods can be used as molecular switches. At the moment, they are largely academic interest.
- Multicomponent quantum dots
Methods of colloid chemistry make it possible to synthesize multicomponent quantum dots from semiconductors with various characteristics, first of all, with a different width of the forbidden zone. This classification is largely similar to the traditionally used in semiconductors.
Alloyed quantum dots
As a rule, the amount of administered impurity is small (1-10 atoms per quantum point with an average number of atoms in a quantum point 300-1000). The electronic structure of the quantum point does not change, the interaction between the impurity atom and the excited state of the quantum point is dipole character and is reduced to the transmission of excitation. The main alloying impurities - manganese, copper (luminescence in the visible range).
Quantum dots based on solid solutions.
For quantum points, the formation of solid solutions of semiconductors is possible if the mutual solubility of the materials in the surround state is observed. As in the case of bulk semiconductors, the formation of solid solutions leads to the modification of the energy spectrum - effective characteristics are superposition of values \u200b\u200bfor individual semiconductors. This approach allows you to change the effective width of the forbidden zone at a fixed size - gives another way to control the characteristics of quantum dots.
Quantum dots based on hetero-transparents.
This approach is implemented in the quantum dots of the type of core-shell (kernel from one semiconductor, the shell from the other). In general, it involves the formation of contact of two parts from different semiconductors. By analogy with the classical theory of heteroxes, you can select 2 types of core-shell quantum points.
Photoluminescent quantum dots.
Of particular interest are photoluminescent quantum dots, in which the photon absorption gives rise to electron-hole pairs, and the recombination of electrons and holes causes fluorescence. Such quantum dots have a narrow and symmetric fluorescence peak, the position of which is determined by their size. So, depending on the size and composition, CT may have fluorescence in UV, visible or IR spectrum.
Quantum dots based on chalcogenides of cadmium depending on its size fluorescent by different colors
For example, quantum dots ZNS., CDS. and Znse fluorescent in UV - area, CDSE.and Cdte. in visible PBS, PBSE. and Pbte. In the neighboring IR areas (700-3000 nm). In addition, from the above compounds can be created by heterostructures whose optical properties may differ from those from the initial compounds. The most popular is the extension of the shell of a more wide-range semiconductor on the kernel from the narrow-earth, for example, on the kernel CDSE. Build the shell of ZNS. :
Model of the structure of a quantum point consisting of a CDSE kernel covered with epitaxial shell from ZNS (Structural type of sphalerite)
Such an admission makes it possible to significantly increase the stability of the CT to oxidation, as well as to increase the quantum fluorescence output by reducing the number of defects on the surface of the kernel. The distinctive feature of the CT is the continuous absorption spectrum (fluorescence excitation) in a wide range of wavelengths, which also depends on the size of the CT. This makes it possible to simultaneously excite different quantum points at one wavelength. In addition, CT have a higher brightness and better photobility compared to traditional fluorophores.
Such unique optical properties of quantum points open wide prospects for their use as optical sensors, fluorescent markers, photosensitizers in medicine, as well as for the manufacture of photodetectors in IR - region, solar batteries high efficiency, ultra-miniature LEDs, white light sources, single-electron transistors and nonlinear-optical devices.
Obtaining quantum dot
There are two basic methods for obtaining quantum dots: colloid synthesis, conducted by mixing precursors "in the flask", and epitaxy, i.e. Oriented growth of crystals on the surface of the substrate.
The first method (colloidal synthesis) is implemented in several versions: with high or room temperature, in the inert atmosphere in the environment of organic solvents or in aqueous solution, Using or without organometallic precursors, using or without molecular clusters, facilitating nucleation. Also used high-temperature chemical synthesis, conducted in an inert atmosphere by heating inorganometallic precursors dissolved in high-boiling organic solvents. This allows you to get homogeneous quantum dots with a high quantum fluorescence output.
As a result of colloidal synthesis, nanocrystals are obtained coated with a layer of adsorbed surfactant molecules:
Schematic representation of a colloidal quantum point type core-shell with a hydrophobic surface. Orange shows a kernel from a narrow-gray semiconductor (for example, CDSE), a red-shell from a wide-range semiconductor (for example, ZNS), a black is an organic shell of surfactant molecules.
Thanks to the hydrophobic organic shell, colloidal quantum dots can be dissolved in any non-polar solvents, and with its corresponding modification - in water and alcohols. Another advantage of colloidal synthesis is the possibility of obtaining quantum dots in subkylogram quantities.
The second method (epitaxy) - the formation of nanostructures on the surface of another material is usually associated with the use of unique and expensive equipment and, moreover, leads to quantum dots, "tied" to the matrix. The epitaxy method is difficult to scale to the industrial level, which makes it less attractive for mass production of quantum dots.
Bosensors using quantum dots. Prospects for their use in clinical diagnostics.
Quantum - A very small physical object, the size of which is less than the boron exciton radius, which leads to the occurrence of quantum effects, such as strong fluorescence.
The advantage of quantum points is that they can be excited by one source of radiation. Depending on their diameter they shine different light, with one source, quantum points of all colors are excited.
At the Institute of Bioorganic Chemistry. Academicians M.M. Shemyakina and Yu.A. Ovchinnikov RAS produces quantum dots in the form of colloidal nanocrystals, which allows them to be used as fluorescent tags. They are very bright, even in a regular microscope, separate nanocrystals can be seen. In addition, they are photo-resistant - capable of glowing for a long time when they exposed to high power density radiation.
The plus of quantum dots is also the fact that, depending on the material from which they are made, you can get fluorescence in the infrared range where biological tissues are most transparent. In this case, the efficiency of fluorescence is incomparable with any other fluorophores, which allows them to be used for visualization. various formations in biological tissues.
On the example of the diagnosis of an autoimmune disease - systemic sclerosis (scleroderma) - the possibility of quantum points in clinical proteomics was demonstrated. Diagnosis is based on the registration of autoimmune antibodies.
In autoimmune diseases, the intrinsic proteins of the body begin to effect on their own bio-objects (on cell walls, etc.), which causes the strongest pathology. At the same time, autoimmune antibodies appear in biological fluids than they used to carry out diagnostics and detect an autoantile.
There are a number of antibodies to scleroderm. The diagnostic capabilities of quantum points were demonstrated using the example of two antibodies. On the surface of polymer microspheres containing quantum points of the specified color, antigens were applied to autoantales (each antigen corresponded to the microspheres). The test mixture contained, except for the microspheres, and secondary antibodies associated with the signal fluorophore. Next, the mixture was added to the mixture, and if it contained the desired autoantibody, a complex was formed in the mixture microsphere - Autoanatelio - Signal Fluoroform.
Essentially, the autoantyolateo was a linker that binds a microsphere of a certain color with a signal fluorophore. Then these microspheres were analyzed using flow cytometry. The appearance of the simultaneous signal from the microspheres and signal fluorophore is evidence that binding occurred, and a complex formed on the surface of the microsphere, including secondary antibodies with a signal fluorophore. At this point, microspheres and signal fluoroform were actually shone, which was associated with the secondary antibody.
The simultaneous appearance of the other signal shows that a detected target is present in the mixture - an autoantylator, which is a disease marker. This is a classic "sandwiche" method of registration, when there are two recognizing molecules, i.e. The possibility of simultaneous analysis of several markers is demonstrated, which is the basis of the high reliability of the diagnosis and the possibility of creating drugs to determine the disease at the earliest stage.
Use as biometers.
The creation of fluorescent marks based on quantum dots is very promising. The following advantages of quantum points can be distinguished in front of organic dyes: the ability to control the luminescence wavelength, high extinction coefficient, solubility in a wide range of solvents, luminescence stability to environmental action, high photostability. It can also be noted the possibility of a chemical (or more biological) modification of the surface of quantum dots, which allows selective binding to biological objects. The right figure shows the staining of cell elements using water-soluble quantum dots luminescent in the visible range. The left figure shows an example of using a non-destructive method of optical tomography. Photography obtained in the near IR range when using quantum luminescence dots in the range of 800-900 nm (the blood transparency window of warm-blood) introduced into the mouse.
Fig.21. Use quantum dots as biometers.
Conclusion.
Currently, medical applications using quantum dots are still limited, due to the fact that the influence of nanoparticles on human health is not enough. However, the use of them in diagnosis dangerous diseases It seems very promising, in particular, on their basis, the method of immunofluorescence analysis is developed. And in the treatment of oncological diseases, it is already used, for example, the method of so-called photodynamic therapy. The nanoparticles are injected into the tumor, then they are irradiated, and then this energy is transferred from them to oxygen, which goes into an excited state and from the inside "burns out" the tumor.
Biologists say that it is easy to design quantum dots, giving a response to any wavelength, for example, in the near infrared spectrum. Then it will be possible to find tumors hidden deep inside the body.
In addition, certain nanoparticles can give a characteristic response with magnetic resonance tomography.
Further researchers look even tempting. New quantum dots, connected to a set of biomolecules, will not only find a tumor and indicate it, but also to supply new generations of drugs to the place.
It is possible that just this application of nanotechnology will be the closest to practical and massive implementation from what we have seen in laboratories in recent years.
Another direction is optoelectronics and new type LEDs - economical, miniature, bright. Such advantages of quantum dots are used here as their high photostability (which guarantees the long functioning of devices created on them) and the ability to provide any color (with an accuracy of one or two nanometers on the wavelength scale) and any color temperature (from 2 degrees Kelvin up to 10 thousand and higher). In a run on the basis of LEDs, you can make displays for monitors - very thin, flexible, with high contrast of the image.
Bibliography.
1.Http: //www.nanometer.ru/2007/06/06/quantum_dots_2650.html
- Tananaev P.N., Dorofeyev S.G., Vasilyev R.B., Kuznetsova T.A. Obtaining CDSE nanocrystals doped with copper // Inorganic materials. 2009. T. 45. No. 4. P. 393-398.
- Oleinikov V.A., Sukhanova A.V., Nabiyev I.R. Fluorescent semiconductor nanocrystals
in biology and medicine // Nano. - 2007. - P. 160 173.
- Snee P.T., Somers R.c., Gautham N., Zimmer J.P., Bawendi M.G., NOCERA D.G. A Ratiometric CDSE / ZNS Nanocrystal pH Sensor // J. Am. Chem. SOC .. - 2006. - V. 128. P. 13320 13321.
- Kulbachinsky V. A. Semiconductor Quantum Points // SOROSOVSKY EDUCATIONAL MANUAL. - 2001. - T. 7. - №4. - C. 98 - 104.
Download:
You do not have access to download files from our server.
Quantum dots are small crystals emitting light with precisely adjustable color value. They significantly improve image quality without affecting the final cost of devices.
Quantum Dot LED - new technology Screens The usual LCD TVs are capable of transmitting only 20-30% of the color range perceived by the human eye. The image on the OLED screen more corresponds to reality, however, this technology is not suitable for the mass production of large displays. But recently, a new one has come to her place, providing the possibility of displaying accurate color values. We are talking On the so-called quantum points. In early 2013, Sony introduced the first quantum dot led, QLED. This year, other models of devices will be launched into mass production, and they will cost as regular LCD TVs and significantly less than OLED solutions. What differ displays produced by new technology from standard LCD screens?
There are no clean colors in LCD TVs.
Liquid crystal displays consist of five layers: the starting point is the white light emitted by LEDs and passing through several filters. Polarization filters located in front and rear, in combination with liquid crystals, adjust the passing light stream, lowering or increasing the brightness. This is possible due to the transistors of pixels that affect how much light will pass through the light filters (red, green, blue). The combination of colors of these three subpixels to which filters are applied, in the end gives a specific color value of the pixel. Mixing colors does not cause problems, but it is impossible to get pure red, green or blue. The reason here lies in filters that pass from a non-one wave of a certain length, but a whole bundle of various wavelengths. For example, orange light also passes through the red light filter.
The LED glows when the voltage is submitted. Due to this, the electrons are transferred from the N-type material into the P-type material. The N-type material contains atoms with excess electrons. In the P-type material, there are atoms that lack electrons. If you get into the last excess electrons, they give energy in the form of light. In the usual semiconductor crystal, this is usually white light formed by a variety of waves of different lengths. The reason for this lies in the fact that electrons can be located at various energy levels. Therefore, the radiated photons have different energy, which is expressed in different lengths of the radiation waves.
Quantum dots - stable light
In displays, quantum dots - crystals are several nanometers as a source of light. In this case, the need for a layer with light filters disappears, because when the crystals are submitted, the crystals are always emitted on them with a clearly defined wavelength, and hence the color value - the energy zone decreases to one energy level. This effect is explained by the tinted dimensions of the quantum point in which the electron, as in the atom, is able to move only in a limited space. As in the atom, the electron of the quantum point may occupy only strictly defined energy levels. Due to the fact that these energy levels depend on the material, the possibility of targeted setting up the optical properties of quantum dots appears. For example, to obtain a red color, crystals from cadmium alloy, zinc and selenium (CDZNSE) are used, the dimensions of which are about 10-12 nm. The alloy of cadmium and selenium is suitable for yellow, green and blue flowersThe latter can also be obtained using nanocrystals from zinc and sulfur compound of 2-3 nm.
Due to the fact that the mass production of blue crystals is associated with great difficulties and costs represented company Sony The TV is not a "pure" QLED TV based on quantum dots. In the rear of the QD Vision, the displays are located a layer of blue LEDs, the light of which passes through a layer of red and green nanocrystals. As a result, they are essentially replacing the frequent filters currently. Thanks to this, the color coverage in comparison with ordinary LCD TVs increases by 50%, but does not reach the level of the "pure" QLED screen. The latter in addition to wider color coverage have another advantage: they allow saving energy, since the need for layer with light filters disappears. Thanks to this, the front of the screen in QLED TVs also gets more light than in ordinary TVs that skip only about 5% of the light flux.
Quantum dots in HD TV
Our eyes are able to see more flowersWhat can display HD TVs. Change this situation Can displays based on quantum dots. Quantum dots are tiny particles with a diameter of several nanometers, which radiate light with one specific wavelength and always with the same color value. If we talk about the light filters used in modern TVs, they provide only blurred colors.
Screens without light filters
In modern TVs white light lED lamps (Illumination) becomes colored due to light filters. In the display based on quantum dots (QLED), the color is formed directly in the radiation source. Brightness adjustment systems through liquid crystals and polarization changes have not been underwent.
 Light cells in comparison
Light cells in comparison
In LEDs, electrons go from the N-type material into the P-type material, while giving an energy in the form of white light with different wavelengths. Filter forms needed color. In QLED TVs, nanocrystals emit light from a certain wavelength, and hence the color.
Wider color coverage
Displays on quantum dots are capable of displaying more natural colors (red, green, blue) than traditional TVs covering a wider color range that is closest to our color perception.
 Size and material determine the color
Size and material determine the color
When the electron (E) is connected to the quantum dot, the energy in the form of photons (P) is released. Using various materials and changing the size of nanocrystals, can be influenced by the value of this energy and, as a result, the length of the light wave.
« Quantum dots are artificial atoms whose properties can be controlled.»
J.I. Alferov, Winner of the Nobel Prize 2000. In physics for the development of semiconductor heterostructures for high-speed and optoelectronics
Quantum dots (CT) are isolated nanoobjects whose properties differ significantly from the properties of the volume material of the same composition. Immediately it should be noted that quantum dots are more like a mathematical model than real objects. And is due to this with the impossibility of forming completely separate structures - small particles always interact with the environment, being in a liquid medium or solid matrix.
To figure out what quantum dots, and understand their electronic structure, imagine an ancient Greek amphitheater. Now imagine that the scene unfascisives, and the audience ranks are filled with the public who came to watch the game of actors. So it turns out that the behavior of people in the theater is largely similar to the behavior of the electrons of the quantum point (CT). During the presentation, the actors move in the arena, without leaving the audience, and the audience themselves follow the action from their places and do not go down on the stage. Arena is the lower completed levels of the quantum point, and the audience ranks are excited electronic levels with higher energy. At the same time, as a viewer can be in any row of the hall, and the electron is able to occupy any energy level of the quantum point, but cannot be located between them. Buying tickets to the idea at the box office, everyone sought to get the best places - as close as possible to the scene. Indeed, well, who wants to sit in the last row, from where the actor's face does not consider even in binoculars! Therefore, when the audience is searched before the view of the presentation, all the lower rows of the hall are filled, as well as in the stationary state of the CT, which has the lowest energy, the lower energy levels are fully occupied by electrons. However, during the presentation, someone from the audience may leave their place, for example, because the music on the stage plays too loudly or just a neighbor is nice caught, and reconcile to a free top row. This is how and in CT, the electron under the action of external influence is forced to switch to a higher energy level, not occupied by other electrons, leading to the formation of the excited state of the quantum point. Probably, you wonder what happens with the empty place at the energy level, where was the electron - the so-called hole before? It turns out that the electron electron is connected with her charge interactions and can go back at any time, as well as the viewer can always change and return to the place marked in his ticket. A pair of "electron-hole" is called "exciton" from the English word "excited", which means "excited". Migration between the energy levels of the CT, similar to the rise or the descent of one of the audience, is accompanied by a change in the electron energy, which corresponds to the absorption or radiation of the light quantum (photon) when the electron is transition, respectively, to a higher or low level. The above-described behavior of electrons in a quantum point leads to a discrete energy spectrum uncharacteristic for macro objects, for which CT is often called artificial atoms in which the electron levels are discrete.
The strength (energy) of the connection holes and the electron determines the exciton radius, which is a characteristic value for each substance. If the particle size is less than the exciton radius, then the exciton is limited in the space by sizes, and the corresponding bond energy varies significantly compared with the volumetric substance (see "Quantum-based effect"). It is not difficult to guess that if the energy of the exciton changes, the energy of a photon emitted by the system changes during the transition of an excited electron to its original place. Thus, obtaining monodisperse colloidal solutions of nanoparticles of various sizes, you can control the transition energies in a wide range of optical spectrum.
The first quantum dots were metal nanoparticles that were synthesized in ancient Egypt for dyeing various glasses (by the way, the Kremlin's rubble stars were obtained along the close technology), although the substrates are grown on the substrates of GAN semiconductor particles and colloidal solutions of CDSE nanocrystals. At the moment, many ways to obtain quantum points are known, for example, they can be "cut" from thin layers of semiconductor "heterostructures" using "nanolithography", and it is possible to spontaneously form in the form of nano-dimensional inclusions of the structures of the semiconductor material of the same type in the matrix of the other. The method of "molecular-beam epitaxy" with a significant difference in the parameters of the elementary cell of the substrate and the sprayed layer can be achieved on the substrate of pyramidal quantum dots, for the study of the properties of which the Nobel Prize was awarded to the Academician J.I. Alferov. Controlling the conditions for the synthesis processes, theoretically, you can get quantum points of certain sizes with specified properties.
Quantum dots are still a "young" object of the study, but wide prospects for their use for the design of lasers and new generation displays are already quite obvious. The optical properties of the CT are used in the most unexpected areas of science that require the rebuilt fluorescent properties of the material, for example, in medical studies with their help it turns out to be able to "highlight" patients with tissue. People who dream of "quantum computers" see in quantum points of promising candidates for building qubits.
Literature
N. Kobayashi. Introduction to Nanotechnology. M.: Binin. Laboratory of Knowledge, 2007, 134 p.
V.Ya. Demikhovsky, G.A. Vugalter physics of quantum low-dimensional structures. M.: Logos, 2000.
Any substance of microscopic size is nanoparticle, material used by nanotechnology researchers to develop and create new technologies based on the use of elements in this tiny form. We read carefully, because it will be necessary to delve a little in the essence of the text.
Quantum dots are nanoparticles made from any semiconductor material, such as silicon, cadmium selenide, cadmium sulphide or India arsenide, which are glowing in a certain color after lighting with light.
The color they light depends on the size of the nanoparticles. By placing quanta different size You can achieve red, green and blue in each pixel display screen, which will make it possible to create a full range of colors in these pixels (any existing color is obtained by mixing these colors).
When quantum dots are illuminated by the UV light, some of the electrons get sufficient energy to free themselves from atoms. This ability allows them to move around nanoparticles, creating a conduction zone in which electrons can freely move on the material and carry out electricity.

When electrons are lowered to an external orbit around the atom (valence zone), they emit light. The color of this light depends on the difference of energies between the conduction zone and the valence zone.
The smaller the nanoparticle, the higher the energy difference between the valence zone and the conduction zone, which leads to a deeper blue color. For greater nanoparticles, the difference in energy between the valence zone and the conduction zone is lower, which shifts the glow towards the red.
Quantum dots and displays
For LCD displays, the advantages are numerous. Let's consider the most important and interesting featureswho received LCD screens from quantum dots.
Higher peak brightness
One of the reasons why manufacturers are so "flashing" from quantum dots is the ability to create screens with a much greater peak brightness than when using other technologies. In turn, increased peak brightness gives much greater opportunities for using HDR and Dolby Vision.
Dolby Vision is a video standard that has an extended dynamic range, that is, a very big light difference between the brightest and darkest point on the screen, which makes the image more realistic and contrast.

If you do not know, the developers are constantly trying to play Lord God and create what he created (well, or who all this created it around us, maybe the universe?), Only transfer it to the screen.
That is, for example, the usual sky on a clear day has a brightness of approximately 20,000 NIT (units. Dimensions of brightness), while the best TVs can provide brightness of about 10 less. So, the Dolby Vision standard is still ahead of the planet all, but to the creator they are still very far :)
Accordingly, the screens on quantum dots are one more step to a brighter image. Perhaps we will someday be able to see almost real dawn and / or sunset, and maybe other unique wonders of nature, without leaving home.
Best color reproduction
Another great advantage of quantum dots is to improve color accuracy. Since each pixel has a CT red, blue and green, it makes it possible to access the full palette of colors, which, in turn, allows you to achieve an incredible number of shades of any color.
Improved battery life of mobile devices
Screens on quantum dots promise to have not only excellent image quality, but also have exceptionally low power consumption.
Quantum dots and samsung QLED
Televisions on quantum points from Samsung, or simply, in fact, not quite on quantum points in the correct understanding of this technology. QLED is rather a hybrid, something mean between quantum dots and LED screens. Why? Because in these TVs is still used. neon lightsAnd in the present screen on quantum points the light should be created by exactly points.

Therefore, even if new TVs from the South Korean giant and show better than ordinary LED screens, they are still not televisions on quantum dots, and TVs with quantum dots instead of the light filter.
Comments:
| Ivan Ivanovich |
In order to obtain a general idea of \u200b\u200bthe properties of material objects and laws, in accordance with which "lives" the usual macromyr, it is not at all necessary to finish higher educational institution, because every day everyone faces their manifestations. Although B. lately The principle of similarity is increasingly mentioned, supporters of which argue that micro and macromir are very similar, nevertheless, the difference still, there is. This is especially noticeable with very minor sizes of bodies and objects. Quantum dots, sometimes called nanotechnos, are just one of these cases.
Less less
Let's remember classic device Atom, for example, hydrogen. It includes the core, which, thanks to the presence of a positively charged proton in it, has a plus, that is, +1 (as hydrogen is the first element in the Mendeleev table). Accordingly, at a certain distance from the kernel there is an electron (-1), forming an electronic shell. Obviously, if you increase the value, it will entail the attachment of new electrons (Recall: In general, the atom is electrically neutral).
The distance between each electron and the core is determined by the energy levels of negatively charged particles. Each orbit is constant, the total particle configuration determines the material. Electrons can leap from one orbit to another, absorbing or highlighting the energy by means of photons of one or another frequency. In the most remote orbits there are electrons with a maximum energy level. What is interesting, the photon itself shows a dual nature, alternating at the same time as a massless particle and electromagnetic radiation.
The word "photon" greek originIt means "light particle". Therefore, it can be argued that when changing the electron of its orbit, it absorbs (highlights) a quantum of light. In this case, it is appropriate to explain the meaning of another word - "Kvant". In fact, nothing is difficult. The word happened from the Latin Quantum, which is literally translated as the smallest meaning of any physical value (here - radiation). Let us explain on the example what a quantum is: if there was a milligram when measuring the weight of the weight, it could be so called it. That's so simply explained, it would seem a difficult term.
Quantum Points: Explanation
Often, in textbooks, you can find the following definition for the nanochka - this is an extremely small particle of any material, the dimensions of which are comparable to the value of the radiated electron wavelength (the full spectrum covers the limit of 1 to 10 nanometers). Inside it, the meaning of a single negative charge carrier is less than outside, therefore the electron is limited in movements.
However, the term "quantum dots" can be explained otherwise. The electron, which absorbed photon, "rises" to a higher energy stage, and in its place "shortage" is formed - the so-called hole. Accordingly, if the electron has -1 charge, then the hole is +1. In an effort to return to the previous steady state, the electron emits a photon. The connection of charge carriers "-" and "+" In this case, the name of EXITON and physics is understood as a particle. Its size depends on the level of absorbed energy (higher orbit). Quantum points are just these particles. The frequency of the radiated electron of energy directly depends on the size of the particle of this material and the exciton. It is worth noting that the color perception of light with a human eye is a different