The role of water in chemical processes. Presentation on the topic: "The role of water in chemical reactions
To see a presentation with artwork and slides, download the file and open it in PowerPoint on your computer.
Slides text content:
Come on, take off your hat quickly! I am the daughter of the space dad. And omnipresent and light, - I am ice, I am sweat, I am clouds. I am frost, tea, broth, fog, River, stream and ocean. When I am angry, I boil; And from the frost - I freeze. Water "The role of water in chemical reactions»Grade 11 Author of the presentation: teacher 1 qualification category MAOU "Gymnasium No. 1", Syktyvkara Yurina Alexandra Viktorovna The surface of the Earth is 71% water 1. The multifaceted role of water in the conversion of H2O substances as a reaction medium Reactions take place in solutions By solubility in water Soluble (more than 1 g of a substance is dissolved in 100 g of water) Alkaline hydroxides Me; Almost all nitrates; All monosaccharides; Lower alcohols Carboxylic acids Slightly soluble (from 0.01 to 1 g of substance is dissolved in 100 g of water) Ca (OH) 2; BaF2 and AlF3; Silver and lead sulfate Aniline and phenol Practically insoluble (in 100 g water dissolves less than 0.01 g of substance) Almost all phosphates and carbonates; All hydrocarbons; Higher alcohols Aldehydes Carboxylic acids H2O as a reagent Water splitting under the action of light (photolysis) in the light phase of photosynthesis; A necessary reagent in the reactions of the cleavage of complex biopolymers (hydrolysis reactions). During the reactions of the formation of biopolymers, polymerization, water is released H2O as a dissociation factor Water molecules are polar, therefore electrolytes easily dissociate in water H2O as a catalyst H2O * If you just mix them, the reaction does not go - if you add a drop of water, the reaction will immediately start and will go roughly. Interaction of dry powders of aluminum and iodine. 2Al + 3J2 = 2AlJ3 + Q H2O as a carrier of substances All liquid media consist of more than 90 - 98% water Carries O2 throughout the body, nutrients, hormones, etc. Blood Delivers CO2 and decay products to the places of their removal from the body 2. Processes occurring during dissolution Destruction of the crystal lattice; Diffusion of dissolved substances; Chemical process - hydration (interaction of molecules of a dissolved substance with H2O) a) hydration reaction of acetylenab) CuSO4 + 5H2O = CuSO4 * 5H2O blue crystals white 3. Electrolytic dissociation Electrolytes are substances that decompose into ions (dissociate) in solutions. ) (The degree of dissociation tends to zero) All alkalis; All salts; Acids: HCl, HNO3, H2SO4, HClO4, HBr, HJ, CF3COOH, etc. Many inorganic acids (H2S, H2CO3, HCN); Almost all organic acids. Acids These are electrolytes dissociating into hydrogen cations and anions of an acid residue These are electrolytes dissociating into hydrogen cations and anions of an acid residue HCl = H + Cl HCOOH H + HCOO + - + - Bases These are electrolytes dissociating into metal cations (ammonium or organic base) and hydroxide - anions KOH = K + OH CH3NH2 * H2O CH3NH2 + OH + - + - Salts These are electrolytes dissociating into metal cations (ammonium or organic base) and anions of the acid residue Al2 (SO4) 3 = 2Al + 3SO4 NH4NO3 = NH4 + NO3 CH3COONa = Na + CH3COO 3+ 2- - + + - Homework 1. item 17 - read. Learn notes in notebook 2. Reactions of ion exchange (write down the full and abbreviated ionic equations): A) K2S + 2HCl = B) K2S + NaNO3 = C) K2SO4 + Pb (NO3) 2 = 3. Restore the molecular equations: A) Fe + 3OH = Fe (OH) 3B) 2H + S = H2SB) 3Ca + 2PO4 = Ca3 (PO4) 2 + 2- 3+ - Used literature Chemistry. Grade 11. Basic level: textbook. for general education. institutions / O.S. Gabrielyan. http://kozlenkoa.narod.ru/indexlessons.htmhttp://yandex.ru/images/searchhttp://forum.xumuk.ru/index.php?showtopic=97449
Attached files
MBOU "Molodkovskaya secondary school"
Mglinsky district, Bryansk region
Chemistry teacher: Shtyrkhunova Tatyana Aleksandrovna
Summary of a chemistry lesson in grade 11 on the topic: "The role of water in chemical reactions."
The purpose of the lesson: To systematize knowledge about the composition, structure, properties of water, meaning, being in nature, about problems fresh water.
Lesson Objectives: 1. Examine the various areas of the role of water in chemical reactions.
2. To develop logical thinking, skills of obtaining information from various sources. To instill skills of self-organization and self-esteem.
3. To educate students about water as an irreplaceable substance in the life of all living organisms.
Lesson type: Combined lesson.
Equipment used:computer, multimedia projector, cards, solubility table, a number of active metals.
Used CRC:computer presentation.
Formed chemical knowledge, abilities, skills of students: systematization of students 'knowledge about the prevalence of water in nature, physical and chemical properties, applications of water, expanding students' knowledge about the role of water, about environmental issues fresh water.
Formed competencies:
educational and cognitive competence: the development of skills to compare, analyze, prove, be able to solve the following life-practical tasks: the ability to assess the state environment, putting forward their ideas for the protection water resources native land;information competence: development of the ability to analyze and select the necessary information, the ability to prepare and make messages, the ability to use the Internet to search for educational information.
During the classes.
1. Organizational moment.
Checking d.z.
They answer a number of questions on the topic: Reversible and irreversible reactions. Chemical equilibrium.
2. Introductory speech of the teacher.
Teacher. Guys, in today's lesson we will get acquainted with an amazing substance, its physical properties, anomalies, being in nature, value and natural reserves. I invite you to make a trip to the Land of wonderful transformations and conduct our lesson under the motto "The joy of seeing and understanding is the greatest gift of nature." (A. Einstein) What citizen of this country will we meet today?
Slide 1. I am reading a poem about water.
So, what substance will be discussed in the lesson?
I formulate the topic of the lesson, introduce the purpose of the lesson.
“Water ... You have no taste, no color, no smell, you cannot be described - you enjoy you, not knowing what you are.
You are not just necessary for life, you are life itself. You are deity, you are perfection, youthe most great wealth in the world "
These words were written about water by a French writerAntoine de Saint-Exupery.
And our lesson is dedicated tothe most familiar and at the same time the most mysterioussubstance - water.
We write down the topic of the lesson in notebooks: « The role of water in chemical reactions ”.
Introductory word from the teacher about water:
Water is familiar and incomprehensible, amazing, paradoxical, mysterious, unpredictable. There are no number of epithets that could fully characterize this unique substance. She was and remains a muse, a source of inspiration for poets, artists, composers, scientists who have been solving the secrets of this great creation of nature for many years and never cease to be amazed at what they find out.
Acquaintance with slides "2-6.
Teacher. You know from your geography course that water is the most abundant substance on Earth.
Message 1. "Water on Earth"
Water is the most important, the most important substance in the world around us. She is both familiar and unfamiliar, and known, and mysterious ... Natural water! Look up to the sky and you will see clouds or clouds that stretch for miles. How easily they float! But don't imagine that they are weightless. The mass of 1 km3 of clouds is about 2000 tons, and in the Earth's atmosphere water vapor is about 12 300 km3, and this is also natural water... Everyone knows the currenton land streams: streams, rivulets, rivers. Sometimes they spread widely and freely over the plain, sometimes they form powerful rapids, fall from a height of tens and hundreds of meters in waterfalls, carrying their waters into the oceans and seas. About 71% of the surface of our Earth is covered by the World Ocean, accounting for about 97% of all surface waters and half of the lithospheric.
So much or little water on Earth? Very little! Of the total volume of the Earth, water accounts for about 2.5 billion km3.
Water shell The earth is 1.5 billion km3, and the rest is located in the deep layers of the earth's crust. Most of the water is salty, but suitable for life, fresh, only about 5 million km3. A person, however, every year needs more and more fresh, pure water... Humanity is threatened by a crisis due to water pollution. Some countries are already experiencing a shortage of clean fresh water and are forced to import it from abroad. Water must be protected.
Teacher. You know from your biology course that water is inextricably linked to the existence of life on Earth.
How much water is in the human body?(65%- 75%)
Do you know that the brain contains80% water.6 slide.
Message 3. "Water in a living organism"
All life on our planet is 2/3 water. Microorganisms rank first in terms of water content in a living organism, plants rank second, animals rank third, and humans last.
Bacteria are 81% water, spores 50%, animal tissue an average of 70%, lymph 90%. The richest tissue in water is the vitreous body of the eye, which contains up to 99% moisture.
Water in the body performs several functions: the substances dissolved in it react with each other, water helps to remove metabolic waste, serves as a temperature regulator, being a good heat carrier, as well as a lubricant.
In living organisms, water can be synthesized in tissues. So, for example, in a camel, fat in the hump, being oxidized, can give up to40 liters of water. A person, drinking 2.5 liters of water per day, daily washes the stomach with 10 liters of liquid and evaporates 0.7 liters of water.
2 .The structure of the water molecule andphysical properties (7 slide and 8).
(frontal survey)
Slide 9. Find chemical errors in the text (we do the work frontally).
Teacher: Your answers have shown that you are ready for the journey ahead.
Yes, the role of water in chemical reactions is multifaceted. Slide 11.
12 slide Water - reaction medium.
Water is the best solvent in the world; it dissolves many solid, liquid and gaseous substances. Almost all elements are contained in the water of the seas and oceans periodic system, billions of tons of metal ores. Therefore, ocean water is called "liquid ore"; sodium, chlorine, gold, uranium, and various salts are extracted from it.This property of water has great value for nature, almost all chemical transformations on Earth, including in living organisms, occur in water, in its presence, or with its participation.
Due to the high polarity of the molecules, water is able to dissolve many substances with ionic and covalent polar bonds.
In relation to water, substances are divided into:
1) soluble2) insoluble3) slightly soluble Slide13.
Consider the table of solubility of substances.
Using the solubility table,write examples of substances on the chalkboard.
Ba (OH) 2,KCl,BaSO4, CaSO4,NaNO3, CuSO4, HNO3, AgCl,FeS, Ca (OH) 2
Slide 14. Water dissociation factor.
Slide 15 Water catalyst.
Let's remember, what is a catalyst?
Slide 16. Water transporter of substances.
Message.
Literally every process in the body takes place with the help of water. Every cell is saturated with water, every chemical reaction takes place with the help of water. This includes the chemical reactions that take place in your body and produce energy, helping you to stay active and have an efficient metabolism. Since water is a component of blood that supplies your body with nutrients, there must be enough water in your blood to deliver nutrients to every cell. Lack of water slows down the metabolic rate.
Slide 17. Water is a reagent. Let's remember what water reacts with?
Water is one of the most reactive substances.
Disciple: From the material we have passed, we know that oxides, hydrides, alkali metals ... and other substances (called them) interact with water
Answers a number of questions.
What is formed when the basic oxide interacts with water? Acidic oxide? .....
Write down the reaction equations in a notebook.
Control over the assimilation of the lesson material.
Teacher: So, we arrived at the final station. Let's do some tasks.
Slide 19. What substances does water react with? Write down the reaction equations. (Work in pairs) Then we check the fulfillment of the task.
Slide 18. Indicate the role of water in the listed processes.
Write it down in a notebook, then check it.
Slide 20 What about in question in this passage? What role does water play?
Lesson summary Slide 21 Christina reads a verse about water.
We conclude: What is water?
Slide 22 Save the water.
Teacher : Remember that the supply of clean water is constantly decreasing, andwe must use water sparingly.
10. Reflection.
Students have three colored cards on their tables, with the help of which we will assess the lesson.
Blue card - "3", yellow card - "4", red card - "5".
How do you assess your knowledge gained in today's lesson?
How do you assess the work of your classmates in the classroom?
How do you rate the lesson as a whole?
Grading a lesson.
11. Homework: paragraph 17, No. 3,4,5.
Olga Golikova
The presentation reflects the physical properties of water; the classification of substances by solubility in water is given; determination of aqueous solutions and electrolytes. The main provisions of the theory of electrolytic dissociation (TED) are presented. The definitions of acids, bases and salts from the point of view of TED are given.The role of water as a vehicle and as a participant in chemical reactions is shown
Download:
Preview:
To use the preview of presentations, create yourself an account ( account) Google and log into it: https://accounts.google.com
Slide captions:
Completed 11th grade student Olga Golikova Presentation on chemistry on the topic: The role of water in chemical reactions
Water is the most common solvent on Earth, largely determining the nature of Earth's chemistry as a science. Most of chemistry, at its inception as a science, began precisely as the chemistry of aqueous solutions of substances. Systematic name: hydrogen oxide Traditional names: water Chemical formula: H 2 O Molar mass: 18.01528 g / mol
Water is a colorless, odorless and tasteless liquid, bale t = 100 ° C, t pl = 0 ° C, p = 1 g / cm 3 (at 4 ° C). Water does not conduct electricity, poorly conducts heat, the specific heat of water is very high. Water is the only substance that shrinks when it hardens. Therefore, the density of ice is less than the density liquid water... This is why ice floats on the surface of the water. Water
Water is unique Chemical substance, the role of which in chemical reactions can hardly be overestimated water Reaction medium Dissociation factor Transport of substances reagent catalyst
A huge number of chemical reactions take place in aquatic environment... According to solubility in water, all substances are conventionally divided into three groups. Solubility of substances in water
These are homogeneous systems consisting of water molecules, particles of a solute and the products of their interaction The process of hydration is the result of the interaction of water with molecules of a dissolved substance Aqueous solutions -
Substances that decompose into ions in solutions dissociate. The ratio of the number of moles of a substance decomposed into ions to the total amount of a dissolved substance is called the degree of electrolytic dissociation All electrolytes are divided into: - strong (the degree of dissociation tends to unity) - weak (in solutions they dissociate very slightly) Electrolytes
Svante August Arrhenius (1859-1927) Swedish chemist and physicist. In 1887 he finally formulated the theory of electrolytic dissociation, in 1887 he explained the deviation of electrolyte solutions from Van't Hoff's laws and Raoult's law (showed the physical meaning of the correction factor i). He created the doctrine of isohydricity, developed the theory of salt hydrolysis. He established the exothermic nature of most electrolyte dissociation processes and the dependence of the rate and completeness of these processes on temperature. Electrolytic dissociation theory
Acids are electrolytes that dissociate into hydrogen cations and acid residue anions; Bases are electrolytes dissociating into metal cations (ammonium or organic base) and hydroxide anions Salts are electrolytes dissociating into metal cations (ammonium or organic base) and anions of acid residue Three types of electrolytes
All liquid media are more than 90-98% water; Blood carries oxygen, nutrients, hormones and other compounds throughout the body, while removing carbon dioxide and decay products Role of water as a vehicle
Hydration reaction (addition reaction) - the reaction of hydration of acetylene compounds with the formation of carbonyl compounds. During the hydration of acetylene, acetaldehyde is formed, in the case of substituted acetylenes, mainly ketones Water as a participant in chemical reactions
It is a source of pure hydrogen and oxygen production. The interaction of hydrogen and oxygen serves as the reaction that allows spacecraft to be launched into orbit Water participates in electrolysis processes
Thank you for your attention
1. What is the structure of a water molecule? What are its physical properties?
2. Expand the integrating role of water in natural science - between chemistry, biology, physics and geography. Is it possible to do this fully enough without structuring and updating the integration problems? 
3. Tell us about the role of water in solving the economic problems of society. 
4. Expand the global problem of mankind - the problem of fresh water on Earth and propose a way to solve it. 
5. Tell us about the role of water in chemical reactions. 
6. Prove that electrolyte dissociation is the result of the hydration process. What role did Russian chemists play in the study of this side of the theory of electrolytic dissociation? 
7. What is the degree of electrolytic dissociation? What groups are electrolytes divided into according to the degree of electrolytic dissociation? Give examples of representatives from each group. 
8. List Chemical properties water. Which of these properties are in practical use? 
9. What are crystalline hydrates? What is the process behind applying plaster casts or making alabaster products? 
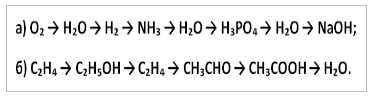
10. Write down the reaction equations with which you can carry out the following transformations: