Phosphorus plus alkali. Phosphorus, production and use
\u003e\u003e Chemistry: Phosphorus and its compounds
The structure and properties of atoms . The next after nitrogen representative of the main subgroup of group V of the Periodic system is the non-metal element phosphorus R. Atoms, compared with nitrogen atoms, have a larger radius, lower electronegativity, and therefore, more pronounced reducing properties. Compounds with an oxidation state of -3 phosphorus atoms are less common than nitrogen (only in phosphides - compounds of phosphorus with metals, for example, Ca3P2, Na3P). More often, phosphorus exhibits an oxidation state of +5 in compounds. But its combination with hydrogen - phosphine PH3 - is the rare case when the covalent bond between the atoms of different elements is non-polar due to the fact that the electronegativity of phosphorus and hydrogen are almost the same.
Phosphorus is a simple substance. The chemical element phosphorus forms several allotropic modifications. Of these, you already know two simple substancesbut: white phosphorus and red phosphorus.
White phosphorus has a molecular crystal lattice consisting of P4 molecules. Insoluble in water, soluble in carbon disulfide. It is easily oxidized in air, and even ignites in powder form.
White phosphorus is very toxic. Special property its is the ability to glow in the dark due to its oxidation. Store it under water.
Red phosphorus is a dark raspberry powder. It is not soluble in water or carbon disulfide. It oxidizes slowly in air and does not ignite spontaneously. Non-toxic and does not glow in the dark.
When red phosphorus is heated in a test tube covered with a cotton swab, it turns into white phosphorus (concentrated vapors), and if you remove the tampon, white phosphorus will flash in the air (Fig. 35). This experiment shows the flammability of white phosphorus.
The chemical properties of red and white phosphorus are similar, but white phosphorus is more chemically active. So, both of them, as it should be for non-metals, interact with metals, forming phosphides:
White phosphorus ignites spontaneously in air, and red burns when ignited. In both cases, phosphorus oxide is formed ^), released in the form of thick white smoke:
4P + 502 \u003d 2P205
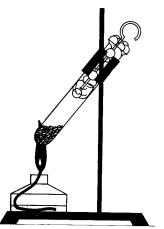
Fig. 35. Experience illustrating the transition of red phosphorus to white
Phosphorus does not directly react with hydrogen, phosphine PH3 can be obtained indirectly, for example from phosphides:
Ca3P2 + 6НСl \u003d ЗСаСl2 + 2РН3
Phosphine - very toxic gas with bad smell. Flammable in air. This property of phosphine also explains the appearance of swamp wandering lights.
Phosphorus compounds
. When burning phosphine or phosphorus, as you already know, phosphorus oxide P205 is formed - a white hygroscopic powder. This is a typical acid oxide with all the properties of acid oxides.
Phosphorus oxide corresponds to phosphoric acid H3P04. It is a solid transparent crystalline substance, readily soluble in water in any ratio. As a tribasic acid, Н3Р04 forms three rows of salts:
middle salts, or phosphates, for example Ca3 (P04) 2, which are insoluble in water, except for alkali metal phosphates;
acid salts - dihydrogen phosphates, for example, Ca (H2P04) 2, most of which are readily soluble in water;
acid salts - hydrophosphates, for example, CaHP04, which are slightly soluble in water (except for sodium, potassium, and ammonium phosphates), i.e., they occupy an intermediate position between phosphates and hydrophosphates in solubility.
In nature, phosphorus does not occur in free form - only in the form of compounds. The most important natural compounds of phosphorus are the minerals phosphorites and apatites. Their main mass is calcium phosphate Ca3 (P04) 2, from which phosphorus is obtained in industry.
The biological significance of phosphorus. Phosphorus is constant an integral part tissues of human organisms, animals and plants. In the human body, most of the phosphorus is bound to calcium. To build a skeleton, a child needs as much phosphorus as calcium. In addition to bones, phosphorus is found in the nervous and brain tissues, blood, and milk. In plants, as in animals, phosphorus is part of proteins.
ATP, adenosine triphosphoric acid, which serves as a collector and carrier of energy, as well as nucleic acids, DNA and RNA, transmitting the hereditary properties of the body, is built from phosphorus that enters the human body with food, mainly with eggs, meat, milk and bread. ATP is most intensively consumed in actively working organs of the body: in the liver, muscles, and brain. No wonder the famous mineralogist, one of the founders of the science of geochemistry, academician AE Fersman called phosphorus "an element of life and thought."
As indicated, phosphorus exists in nature in the form of compounds contained in the soil (or dissolved in natural waters) Phosphorus is extracted from the soil by plants, and animals receive phosphorus from plant foods. After the death of plant and animal organisms, phosphorus again passes into the soil. So the phosphorus cycle is carried out in nature (Fig. 36).
The use of phosphorus and its compounds
. Red phosphorus is used to make matches, phosphoric acid, which, in turn, goes into production phosphate fertilizer and feed additives for livestock. In addition, phosphorus is used to obtain pesticides (remember cans with dichlorvos, chlorophos, etc.). 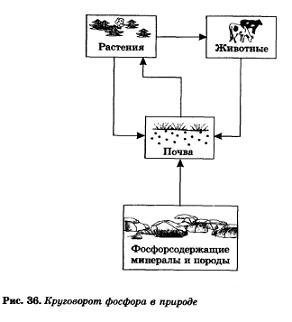
Phosphorus discovery
. Phosphorus was discovered by the German alchemist G. Brand in 1669 and got its name for its ability to glow in the dark (Greek phosphorus - luminiferous).
1. Phosphorus allotropy: white phosphorus, red phosphorus.
2. Properties of phosphorus: the formation of phosphides, phosphine, phosphorus oxide (V).
3. Phosphoric acid and its three rows of salts: phosphates, hydrogen phosphates and dihydrogen phosphates.
4. The biological significance of phosphorus (calcium phosphate, ATP, DNA and RNA).
5. The use of phosphorus and its compounds.
Write the formulas of the three types of sodium salts and phosphoric acid, name them and write down the equations for their dissociation.
Write the reaction equations with which you can carry out the following transformations:
P -\u003e Mg3P2 -\u003e PH3 -\u003e P205 -\u003e H3P04 -\u003e Ca3 (P04) 2
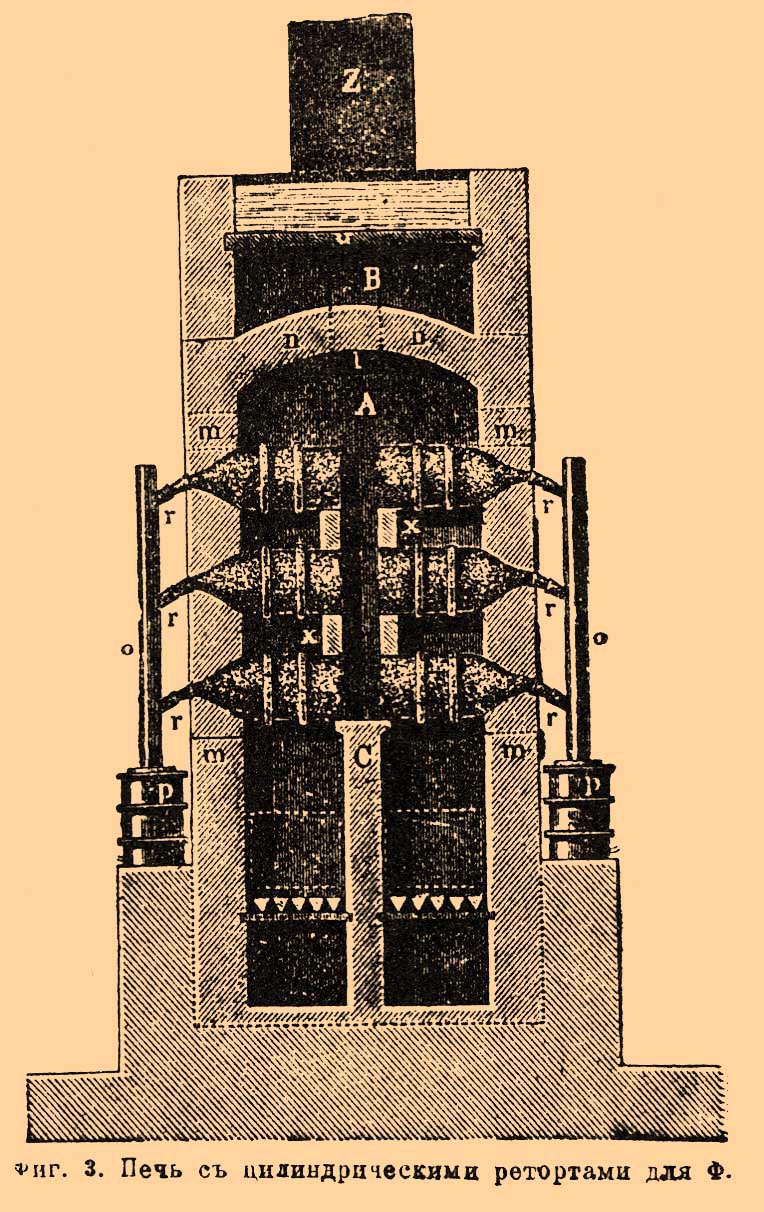
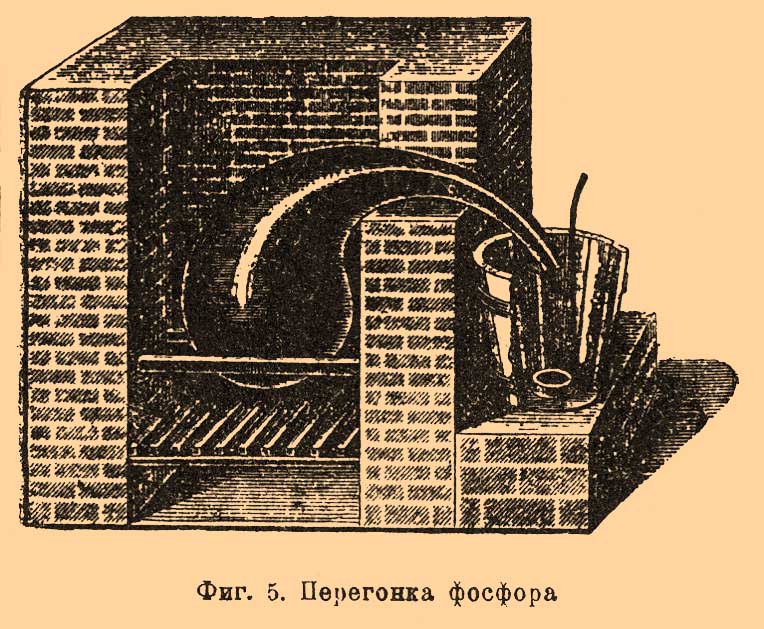
Lesson content lesson summary support frame lesson presentation acceleration methods interactive technologies Practice tasks and exercises self-examination workshops, trainings, cases, quests homework discussion questions rhetorical questions from students Artwork audio, video clips and multimedia photos, pictures, charts, tables, diagrams humor, jokes, jokes, comics parables, sayings, crosswords, quotes Additions abstracts articles chips for curious cheat sheets textbooks basic and additional glossary of terms other Improving textbooks and lessons correction of errors in the textbook updating a fragment in a textbook elements of innovation in the lesson replacing obsolete knowledge with new For teachers only perfect lessons year schedule guidelines discussion programs Integrated lessonsPhosphorus, production and use (tech.). The starting material for the factory preparation of F. is the average phosphoric acid salt of calcium Ca 3 (PO 4) 2, which is widely distributed in nature. In phosphorus plants, it is usually converted to the acid salt of Ca (H 2 PO 4) 2, which is then mixed with coal and calcined; while Ca (H 2 PO 4) 2 first releases water and passes into the metaphosphate salt: Ca (H 2 PO 4) 2 \u003d Ca (PO 3) 2 + 2H 2 O, and the latter is already being restored by coal: 3Ca (PO 3) 2 + 10C \u003d Ρ 4 + Ca 3 (PO 4) 2 + 10CO. Such a preliminary conversion of the average calcium phosphorus salt to acidic is based on the fact that the average salt itself is much more difficult to recover with coal. As can be seen from the above decomposition equation, this way we can distinguish at most 2/3 of the total available F., and 1/3 of it remains in the garbage. To eliminate this drawback, at the suggestion of Wöhler, silica is also introduced into the reaction: 2Ca (PO 3) 2 + 2SiO 2 + 10C \u003d P 4 + 2CaSiO 3 + 10CO, but then the operation requires such a high temperature that can only be economically obtained in electric furnaces, which have recently gained more and more space in technology. The use of electricity for the production of phosphorus is of great importance in that it made it possible to use not the acidic salt of Ca (H 2 PO 4) 2, but directly the average calcium-phosphorus salt of Ca 3 (PO 4) 2; Thus, in addition to completeness of F. separation, when using electric furnaces, the complex operation of converting Ca 3 (PO 4) 3 to Ca (H 2 PO 4) 2 disappears, which takes up a lot of space with conventional phosphorus plants. In this article, we will first consider the commonly practiced methods of fabricating F., and then we will indicate those techniques that are based on the use of electricity. Of all the materials from which acid calcium-phosphorus salt can be prepared (see Phosphorous fertilizers), bones are preferred in phosphorus plants. The denser the bone, than, consequently, it is richer in phosphate salts, the more valuable it is; e.g. horse, bovine and sheep bones are in great demand. Usually they are not subjected to any preliminary operations (for example, to extract fat, etc.), but are directly burned until they are completely converted to ash. Bone firing is often carried out in such furnaces, which make it possible to carry out the operation continuously, and the entire combustion process is carried out at the expense of organic mattercontained in the bones. When firing, measures are taken so that unburned, odorous gases are not released into the surrounding atmosphere. According to Fleck, the device shown in FIG. one. A shaft furnace loaded with bones through a hole closed by a lid but. To open the oven, openings serve bthrough which firewood is introduced and set on fire. These holes have flaps that make it possible to regulate the amount of air entering the furnace, and in addition, already quite burnt material is scoured from the furnace through them. Gases from combustion rise to the top of the furnace from and pass over the firebox here dwhere they burn down completely and then over the hog AT go to the exhaust duct FROM. Over the hog AT There is a series of evaporation tanks with solutions designated for thickening. According to Fleck, for 100 parts of fresh bones taken, 55 parts of completely burnt (white) ash are obtained, in which 80-84% of calcium phosphate, 2-3% of magnesium phosphate, 10-14% of calcium carbonate and fluoride are found. Calcined bones are ground and treated with sulfuric acid to turn the average calcium-phosphorus salt into acidic; this produces gypsum CaSO 4 according to the equation: Ca s (PO 4) 2 + 2H 2 SO 4 \u003d Ca (H 2 PO 4) + 2 CaSO 4. Since the obtained Ca (H 2 PO 4) 2 is soluble in water, and gypsum is poorly soluble, they can be easily separated. The operation is performed in large wooden vats (up to 1.3 m in diameter), lined with lead inside and equipped with a stirrer. For 100 parts of bone ash, according to various sources, from 66 to 90 parts of strong sulfuric acid are taken. Having loaded ash into the vat (up to 140 kg), pour so much boiling water here so that it covers the ash, and then, with constant stirring, sulfuric acid is gradually added. The mass is very foaming from the decomposition of calcium carbonate. Decomposition ends in two days with stirring; water is added to the vat and left to stand still for 12 hours. The settled liquid is siphoned into lead pans for evaporation; the insoluble mass is washed several times with water to possibly completely remove the acidic calcium phosphorus salt, and the washing water is added to the first solution, excluding the last water, which is intended to wet a new portion of bone ash, which is used for decomposition with sulfuric acid. In order to use as little water as possible for such washing (since it then has to be evaporated), washing is carried out in special filtering devices; of these, the simplest are lead-lined wooden crates with a perforated bottom, on which sand is laid, first coarse and then finer, as well as straw and coarse canvas. Such boxes are sometimes placed one above the other in a terrace-like manner, which makes it possible to methodically leach the washed mass. To evaporate a solution of acidic calcium-phosphorus-salt, either the heat of bone-burning and other furnaces is lost, or steam, and the liquid is constantly mixed. This is due to the fact that when the solution is thickened, gypsum is present in the solution in a small amount, which, when the liquid is in a calm state, gives a strong bark on the walls of the pan that does not conduct heat well; with stirring, this does not happen. The thickening of the solution is continued until beats. the weight does not reach 1.4-1.5 (which corresponds to a content of 62% P 2 O 5). The gypsum is separated by filtration, and about 25% of coarse coke or charcoal powder is added to the solution. The mixture is dried in an iron boiler (according to Fleck so that about 5.5% of the water remains) and then calcined in retorts. Retorts made of refractory clay have a pear-shaped or cylindrical shape and are designed to load 6-15 kg of the mixture. It depends on the productivity of the plant, the type of fuel, etc. The arrangement of furnaces for heating retorts is quite diverse. Typically, retorts are not located alone in the furnace, but in groups, sometimes in several rows, one above the other. In FIG. 2 is a cross-sectional view of one of such furnaces. It is arranged for 36 retorts and is 6.6-7 m long, 1.32 m wide and 1.61 m high; she has two fireboxes, which are separated from one another by a low (0.286 meter. above the firebox) wall erunning along the entire stove. Furnace grate but (0.55 m long) only in its front part has grate bars, throughout the rest it is made of bricks. Retorts lie horizontally on either side of the longitudinal wall eleaning on it with its back. The flue gases, covering the retorts, go into the hog d (height 0.175 m and 0.695 m wide.) through the openings in the arch and sent to the exhaust pipe g; at the same time, behind the oven they pass under frying pans, where solutions of calcium phosphorus salt are evaporated. The throat of each retort exits from the furnace to the outside (through the wall, collapsible for each pair of retorts) and connects to the receiver for condensation F.; the latter consists of two clay glazed caps with tubes, with which they are connected with each other and with the throat of a retort. Each cap has a height of 0.18 m, diam. 0.154 m and stands on a round stand 0.01 m high. and 0.24 m diam. filled with water. In FIG. 3 shows a furnace for cylindrical retorts; She also has 2 fireboxes. Retorts lie on one and the other side of the middle wall FROM in three rows, the bottom row resting on its back on the wall itself, while the upper rows are supported by gaskets x. Flue gases rise to the roof n and through the holes l go to the hog AT and then into the pipe Ζ
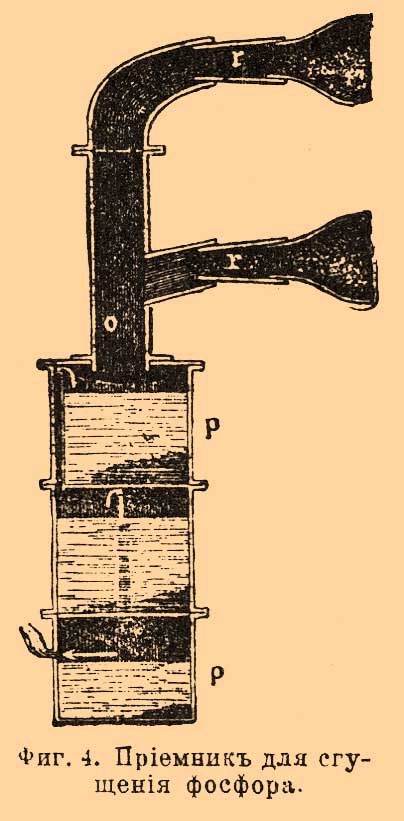
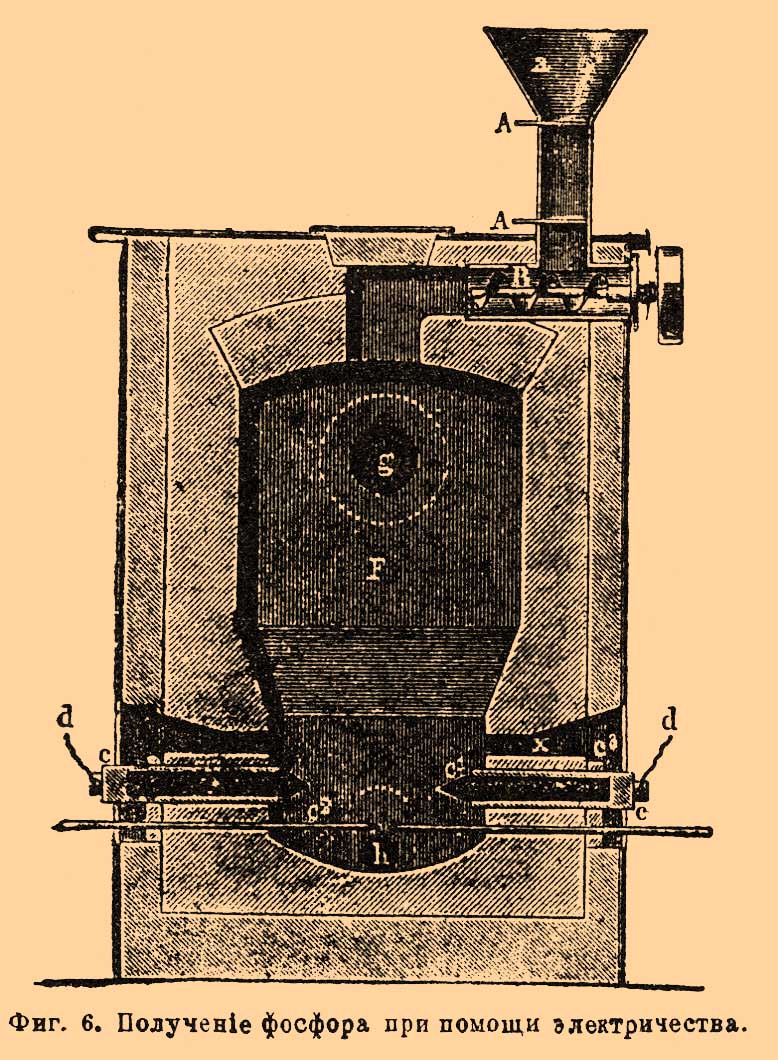
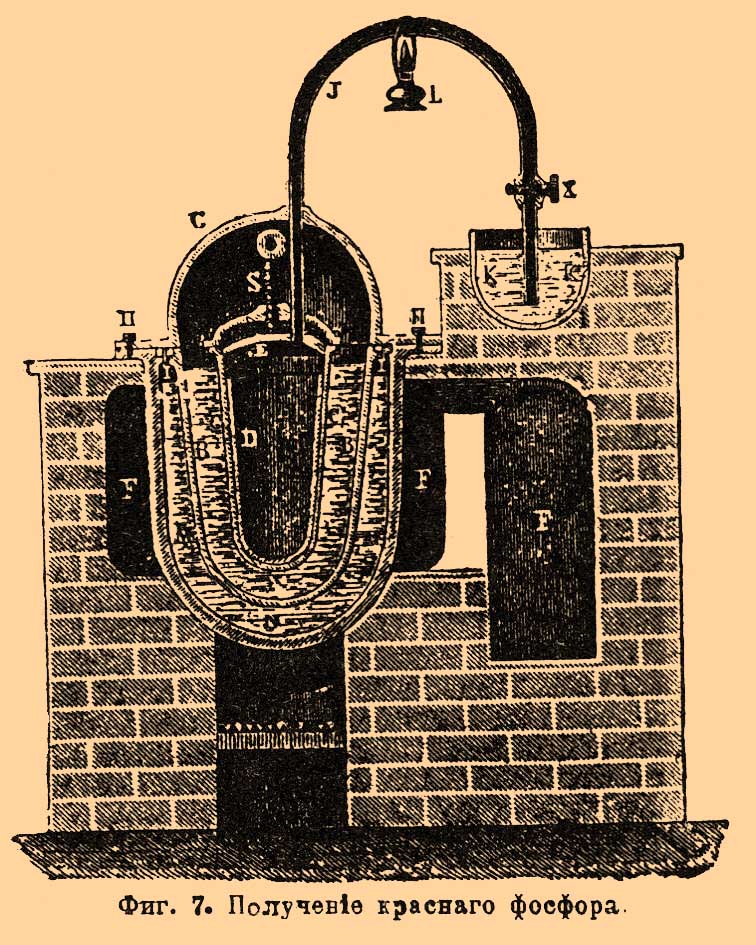
. Throats r every three retorts are connected to one common receiver op (separately Fig. 4 for two retorts) made of enameled iron. It consists of a vertical pipe about with lateral nozzles, which include tips mounted on the throat of the retort, and from the cylindrical part ppbroken into three compartments. F. couples through the pipe about enter the upper compartment, filled with water, where for the most part they condense, and F. gathers under water. Non-condensed vapors and gases, as shown in FIG. arrow, they go to the middle compartment, also filled with water, and then through the tube located here in the middle pass into the lower compartment (with water) and go out, igniting, to the outside. F. is collected under water in all three departments. There are other types of devices, both furnaces and retorts and receivers. The operation itself is carried out as follows: retorts are loaded and smeared into the furnace; their throat is inserted into the receivers and coated with clay or other putty so that there are no gaps through which the F. couples exit; then they begin to gradually warm the oven (with rapid heating, the retorts may crack). The temperature rises gradually, and F. begins to distill; along with it gases that are unpleasantly smelling and harmful to the health of workers (phosphorous hydrogen, carbon monoxide, etc.) are released from the receivers; therefore, the receivers try to solitary and ventilate the rooms where they are located. When stripping, F. is observed so that there is no blockage in the receivers, and from time to time they are cleaned with an iron rod. After a day, the race is greatly weakened, which is noticed by the flame of gases leaving the receivers; after 1 1/2 - 2 days, it completely stops, and then the heat is gradually reduced in the oven. When the oven cools down, the receivers are separated from the retorts and attach to them the end of the throat of the retort, where F. is usually located; the furnace wall is disassembled, the retort is removed and usually thrown to the side, after making sure that there is no undecomposed mixture in it. In their place, new loaded retorts are smeared into the furnace. From receivers and debris, a retort is selected by F. under water with the help of special spatulas. Raw F. has a reddish or brownish appearance; according to Fleck, it turns out 15.4%, counting on bone ash. In addition to the impurity of red F., it contains various compounds of F. with carbon, silicon, etc. To purify raw F., it is filtered in some plants and distilled in others. For filtering F. is placed in a suede bag, which is placed in water heated to 50-60 °; molten F. is squeezed out of the bag by a special press. In French factories, molten F. is mixed with coal powder and placed in an iron cylinder with a porous clay partition; letting steam into the cylinder under a certain pressure, press F. through the pores of the septum, and most of the impurities remain with coal and, thus, do not contaminate the porous plate; the remaining coal is mixed with a new batch of F. Distillation of F. is carried out in cast-iron retorts, which are placed in two or three in one furnace (Fig. 5). F. is melted in a copper boiler under water and mixed with sand (1/8 of its weight). When the mass hardens during cooling, it is loaded into retorts, which are first turned over so that, if possible, the glass is all water, and then placed in the oven. The throat of the retort is immersed 1.5-2 cm in a tub of water, where there is a lead cup with an iron handle for collecting distilled F. 5–6 kilograms of raw F. is loaded into the retort. Heating is carried out slowly and uniformly; try to remove water as completely as possible before the start of the race, since it serves as a material for the formation of phosphorous hydrogen, which is constantly released from the retort. When the distillation is over, the furnace is cooled, the retorts are pulled out and cleaned. The first collected portions of F. in color resemble bleached wax, the next have a yellowish-red appearance, and the latter consist of red F. The more accurately the race is conducted, the more white F. is obtained and the more its output. Loss during distillation reaches 10-15%. They clean F. and chemically. For this purpose, according to Readman, it is melted in a lead vessel under water with steam; After draining the water, as far as possible, add 4% of dichromovale potassium salt, mix well for 1/2 hour and then add the same amount of sulfuric acid; the lower oxides of F. are oxidized in this case, and it becomes completely white. If oxidation does not help, F. is subjected to distillation. Purified F. is obtained 8-11% for the taken bone ash. F. goes on sale usually in the form of sticks. For molding it in France, proceed as follows. F. melt under water; then the worker takes a glass tube with an iron tip equipped with a faucet, and, having immersed it in F., he pumps it with his mouth to the faucet, which then closes; The tap serves to ensure that molten F. cannot get into the mouth. A worker has up to 20 such tubes. The tubes are cooled, and from them through the tap hole F. is pushed out with an iron rod. One worker can prepare in this way up to 100 kg F. In English factories this operation is conducted in a safer way for workers. The molding apparatus consists of a copper quadrangular box placed in an iron boiler with water; it contains F., which melts when the water in the boiler is heated. Two horizontal brass tubes are inserted into the bottom of the box, polished inside. These tubes, passing the walls of the boiler, enter at their end (up to 3 cm) into a long (2-3 meters) box through which current flows cold water. F. in the tube freezes, but remains quite soft and viscous. Before starting work, the bent end of the iron wire is inserted into these tubes, which envelops the frozen F. By pulling the wire, you can gradually pull out such a long F. rod from the tube, as far as the box dimensions allow (up to 2-3 meters). When it is no longer possible to extend further, F. is cut off almost at the brass tube itself, however, at the same time, a small piece of it is left, for which they continue to pull the new stick F.; work thus goes on continuously. It can be stopped at night and then continued in the same order. Sometimes F. is made in the form of tiles or circles, which are often made up of separate pieces. Packaging F. requires the observance of many precautions, in the absence of which it can ignite during transportation and storage. F.'s sticks are placed in tin cans weighing 2.5-3 kilograms., Poured with water and carefully corked so that the water could not suck out anywhere, as they are convinced by holding the can for some time on white passing paper. When transporting a large consignment of F., e.g. up to 300 kilos, the corresponding number of tins is placed in a wooden box, upholstered inside by tin; they are then filled with water. Sometimes cans with F. are transported in small wine barrels; at the same time they are filled with water containing a certain amount of alcohol to prevent freezing of water in winter. The barrels are osmolimy, wrapped in hay and lined with canvas. From a friend. production methods F. can indicate the method proposed by Fleck, who had in mind to use organic constituent parts bones for making glue. Fresh bones are crushed to pieces the size of a nut and kept for some time in warm water 50-60 ° to separate the fat; then they are put in baskets and immersed in hydrochloric acid. beats at. 1.05 per week, until they become slightly transparent and flexible; then they are placed in hydrochloric acid. beats at. 1.02 until they are completely soft. The residue that does not dissolve in acid is processed into glue; the solution is evaporated in clay cups, taking advantage of the lost heat of the retort furnaces, until acid calcium phosphate begins to crystallize; then the liquid is cooled in wooden tanks, the released salt is separated from the mother liquor, wrung out, dried at 100 ° and mixed with coal powder. From the mother liquor, first, upon further evaporation, an unclean acidic calcium-phosphorus salt is released, and then with the addition of lime, the remaining phosphoric acid is isolated from it in the form of a medium calcium salt. Subsequently, it is again processed into acid salt together with the residue from the retorts. A significant amount of evaporation, which is introduced with this method, generally pays off a little device gluing production, and he could not displace old way production F. using sulfuric acid. This last method also has many inconveniences. First of all, with it you need to have a sulfuric acid plant near so as not to overpay a lot for its transportation; then it is necessary to have a workshop for the production of retorts that do not last long and give up to 1 / 2-1 kg F.; significant inconvenience is the storage of acidic liquids, evaporation and filtering of solutions, removal of gypsum, etc. Ridlin suggested conducting production F. in an electric furnace. The starting material is natural phosphate; it is ground, mixed with sand and coal and quenched by electric shock. F., as it is formed, disappears and gathers in a special receiver; the remainder gives liquid slag, which flows out of the furnace, and a new portion of a mixture of phosphorite with coal and sand, etc., is replaced in its place. Production is ongoing. An oven used for this purpose in one English factory (at Wednesfield "e) has the following apparatus (Fig. 6). F. - shaft furnace, on top of which is a funnel for loading material but with dampers BUT and screw AT to feed it into the oven. Electricity introduced into the furnace using carbon electrodes FROM"reinforced in metal sleeves FROM. Thin electrodes are used to start the formation of a voltaic arc. C 2 (carbon or metal) that either lie next to the electrodes FROM", or go through them. The resulting vapors and gases exit the hole g, and toxins flow into h. Holes are used to monitor the progress of the operation. x; carbon electrodes are sprinkled through them, in order to more or less protect them from burning out. According to Colardo, a mixture of 310 parts of average calcium phosphorus salt, 260 parts of lime and 160 parts of coal (all in powder) is taken and calcined in an electric furnace. Subject to this proportion of reactants, a mixture of calcium carbonate (carbide) and calcium phosphorus is obtained; only an insignificant part of phosphorus is released in pairs with carbon monoxide. In order not to thicken F. from here, vapors are passed through hot lime, which absorbs F. The resulting mixture of carbide with calcium phosphorus decomposes with water, and acetylene and hydrogen phosphorus are obtained. First, these gases are passed through an incandescent retort or a coal tube filled with coal, where phosphorous hydrogen decomposes into phosphorus and hydrogen, then a series of washing machines pass in which phosphorus precipitates and acetylene is separated from hydrogen (by absorption, e.g., acetone). Hydrogen goes to heat. There is a rather complicated patent by Billaudot, where F. and carbide are simultaneously obtained. The main idea of \u200b\u200bthe patent is to arrange special condensers for vapors of F., where condensation occurs without contact of F. in a heated state with water, as is usually the case, which makes it possible to avoid losses of F. (from its interaction with water) and eliminates the need for further purification F. (by filtration, etc.) Simultaneously with F., calcium carbide is also obtained. Dill (Dill) proposed to decompose a mixture of phosphoric acid with coal powder. To a concentrated solution of phosphoric acid beats. weights of 50-60 ° add 1/4 - 1/5 by weight of coal and such a mixture is loaded into the clay cylinder through a special funnel. The cylinder stands on a stand made of an electricity conductor, through which positive electricity enters the carbon electrode. The other electrode enters the cylinder through the plug at the top; it can be raised and lowered with a screw device. F. couples exit through a branch pipe to the capacitor; operate with a current of 80-150 amperes with a voltage of 120 volts. When a large part of the phytoplankton was released, the current was interrupted for a while, a new portion of the mixture was loaded, and then work continued again. Among other methods of obtaining F., we point out the proposal of Frank and Rossel to perform acid reduction. Calc. aluminum salts in the presence of silica: 3Ca (PO 3) 2 + 10Al + 3SiO 2 \u003d 6P + 5Al 2 O 3 + 3CaSiO 3. At the suggestion of Shearer and Clapp, they take natural aluminum phosphate Al 2 O 3 P 2 O 5, mix it with sodium chloride and charcoal and calcine it in a stream of hydrogen chloride HCl; in this case, a double salt of aluminum chloride with sodium chloride Al 2 Cl 6 4NaCl is formed and F., carbon monoxide CO and hydrogen are released. The reaction can be represented by the following equation: Al 2 O 3 P 2 O 5 + 4NaCl + 6HCl + 8C \u003d Al 2 Cl 6 NaCl + 8CO + 3Η 2 + 2P. The materials taken should be well ground. Calcination is carried out first at about 10 o’clock with dark red heat until carbon monoxide and hydrogen cease to be released, then the temperature is raised to white heat, and only then F. begins to drive off. The race lasts up to 30 hours, depending on the amount of F. Alfred Kraus proposed calcining a mixture of phosphates with iron ores, e.g. hematite, and thus prepare phosphorous iron; the latter is then fused with pyrite; F. thus volatilizes and thickens, and remains sulfur dioxide. It is left to mature in the open air and is gradually oxidized to iron sulfate, etc. White F. usually contains an admixture of arsenic (0.5-3.5 °); sulfur, carbon, calcium, and others are found in it. For production in large sizes red phosphorus is often used by the method proposed back in 1845 by Schrötter. In the oven F (Fig. 7) two boilers are placed in one another, the gap between which is filled with an alloy of tin with lead N (in equal amounts). In the internal boiler M there is a cover Gbolted NN to the edges of the external boiler. In the boiler M there is sand Bin which the third portable boiler is placed FROM with glass or porcelain receiver R. In his cap E iron or copper curved tube ends Jthat goes through the cover G and its other end immersed in water or mercury in a vessel k; she has a crane x. Under the tube J there is an alcohol lamp to warm it in case of clogging F. Cover E held in place by a spring Swhich, with sudden high pressure inside the boiler FROM moves and the cover can be lifted. The operation of turning white F. into red with this unit is very simple. Dry pieces of F. put in a boiler FROMput the cover in place Ε
and G and begin to gradually heat up. Boiler air FROM comes out of the handset J. The temperature is raised to 260 ° (it is determined by a thermometer dipped in molten metal N) and hold it for several days (up to 10), after which the furnace is cooled, having previously closed the tap x and the formed red F. is broken out. The Schroetter apparatus was subjected to numerous modifications. Coignet in Lyon performs the same operation in one iron boiler. Obtained by the described method, red F. usually contains traces of white F. In one sample of raw red F. Fresenius and Onion (Luck) found white F. 0.56%, phosphorous acid. 1.302%, phosphoric acid. 0.880%, water and other impurities 4.622% and red F. 92.63%. To remove white F. use various means. Raw red F. is subjected to carbon disulfide treatment, which dissolves white F. without touching red. F. is isolated from this solution by distillation of carbon disulfide, which then goes into business again. Sometimes they make F. slowly oxidize in air to phosphoric and phosphorous acid and then wash it with water. At the suggestion of Nickles (Nickles), F. agitate in a solution of calcium chloride ud. weight 1.95; white F., as lighter, floats to the surface, and red gathers at the bottom. It is then washed with water and dried. The main mass produced in technology F. goes for the production of matches; some of it goes to get phosphoric anhydride, for the preparation of explosives, etc. S. Vukolov. Δ. Phosphorus (Medic.) - Of the two modifications F. red, or amorphous, insoluble in tissue fluids and physiologically therefore completely indifferent, even when using large doses; yellow white crystalline, or official, F. dissolves, although in very small quantities, in water, alcohol, fats and bile and has pronounced toxic properties. In 100 parts warm water dissolves 0,00027 F.; the solubility in intestinal fats and bile is 0.01-0.026 per 100. The effect of official F. on the body seems completely different, depending mainly on the size of the dose and duration of use. With the introduction of very small doses for a long time, F. exhibits an irritating effect almost exclusively on bone-forming substances, and this irritation does not lead to the degeneration of the affected tissues, but to their depletion. Wegner, giving weeks of young growing animals such small amounts of F. that are unable to cause disorders of the general condition, found in the blood of experimenters the highest degree wonderful changes. It turned out that in all those places where, under normal conditions, a broad-loop spongy bone substance with a rich content of red brain tissue develops from cartilage, under the influence of F., a completely uniform dense and strong tissue is obtained, in appearance, microscopic structure and chemical composition (in the ratio of organic to inorganic substances, in the content of phosphate salts) is no different from the compact bone tissue of the cortical layer of tubular bones. The spongy bone substance formed earlier, before feeding F., at the same time remains completely unchanged. Bone tissue formed from the periosteum, i.e., that which causes bone growth in thickness, undergoes a similar process of thickening, although less pronounced. However, if too small amounts of F. are administered to the animal for a long time, then the spongy substance remaining unchanged is absorbed, and subsequently the artificially formed bone substance is subjected to the same rarefaction process with the formation of red brain tissue in both cases. These are the phenomena during the repeated administration of very small doses of F. Observations of various researchers have further established that if F. is administered in moderate but gradually increasing doses or if the phosphorus vapors are inhaled frequently, as is the case in match factories, the result is very pronounced inflammatory changes in the bones, leading to their necrosis. The so-called name observed by workers in match factories. phosphorus necrosis of the jaws comes usually from carious teeth or ulcerated gums (see). The data obtained by Wegner, confirmed by other researchers, served as the starting point for the therapeutic use of very small doses of F. in some pathological conditions of the skeletal system, especially with a delay or insufficient development of the skeleton in childhood (with rickets), with osteomalacia, with insufficient ossification of corns, after fractures, etc. Most observers (Kassovitz, Rauchfus, Mandelstam, Shabanova, etc.) note the very favorable effect of F. on the general condition of children suffering from English disease, on their departure limbs, the symptoms of laryngospasm so formidable in rickets. Adults are given 0.0003 grams to 0.001 grams per reception 1-3 times a day (the highest dose per day is 0.005 grams), children are not more than 0.0005 grams per day. If you exceed the indicated cautious doses, then poisoning, The reason for it is rarely negligence, for the most part - an attempted suicide. For the latter purpose, they usually use the heads of phosphorus matches, less often - phosphorus paste used to kill rats (a mixture of F. with ordinary dough, with added fat). In the 50-70s. of the last century, when the Swedish matches prepared with the help of harmless red F. were not yet in use, poisoning of F., especially in Germany and France, was a fairly common occurrence. In France in 1851-71 Among 793 poisonings, 267 (38%) fall in F. poisoning. Large whole pieces F. can, without dissolving, pass through the intestine without much harm. Poisoning seizures are detected only a few hours after the introduction of the poison, expressed in the sensation of thirst, in severe pain in the stomach, in vomiting with a garlic odor and masses glowing in the dark. With relatively small receptions of F., this is limited to this, especially if most of the poison was removed by vomiting or by artificial pumping of the contents of the stomach. In more serious cases, the described local phenomena first subside for 3-4 days, but after this apparent lull, poisoning develops into a difficult picture of an eating disorder. Gastrointestinal disturbances resume, the liver enlarges, the skin and sclera become yellowish, the general condition worsens, cardiac activity becomes more and more frustrated, the patient complains of muscle pain and general weakness, and from all the mucous membranes, from the nose, intestines, and uterus bleeding artificially caused and menstrual bleeding are very profuse and usually do not stop. The amount of urine excreted gradually decreases, bile pigment, bile acids, protein open in it, and in the last days diseases of the renal epithelium, blood and fat cylinders. The excretion of nitrogen by the urine increases very significantly, often three times against the norm, the urea content, on the contrary, decreases very sharply, in severe cases, meat-lactic acid, peptone, often leucine and tyrosine are found in the urine. Consciousness for the most part is preserved until the very end, in other cases - one, two days before death, brain disorders, drowsiness, delirium, convulsive phenomena occur. Death usually occurs 7-8 days after poisoning. With the introduction of poison in a very large dose, the patient can die within a few hours from heart failure. However, there are known cases of recovery, which lasted 4-6 weeks and was accompanied by increased urine output. Postmortem anatomical diagnosis characterized by 1) numerous hemorrhages in the skin, subcutaneous and intramuscular tissue, in the mucous membranes, in the peritoneum, in the pleura and 2) fatty degeneration of the liver, kidneys, heart, pancreas, glands of the mucous membranes of the stomach (gastroadenitis) and intestines, skeleton muscles and walls vessels. The essence of pathological changes in acute F. poisoning is a deep metabolic disorder, which is based on a decrease in oxidative processes in the body and increased protein breakdown. According to Bauer, under the influence of F., the emission of carbonic acid decreases by 47%, and the absorption of oxygen by 45%. Due to insufficient oxidation, protein substances do not turn into ordinary end products, but form intermediate substances from which diffusible ones (lactic acid, peptone, etc.) are excreted in the urine, while colloidal, like fats, are deposited in tissues. Jaundice is due to the pressure produced by the enlarged fat-regenerated liver cells in the bile ducts. The cause of bleeding lies in the fatty degeneration of the walls of all, even the smallest, vessels, and in the blood that has come out of the vessels during F. poisoning and has very low coagulability. Treatment of acute poisoning F. Perhaps early mechanical removal of the poison using a gastric pump or vomit. The best vomit is copper sulfate; it acts simultaneously as an antidote. It is given 0.2 g. every 5 minutes until vomiting occurs, and then continue to give 0.05 grams in 1/4 hours as an antidote. Copper covers F. particles with a layer of sparingly soluble and therefore inactive phosphorous copper. In view of slow absorption of F. from intestines it is possible to count also on laxatives; however, it is necessary to carefully avoid oily laxatives, as well as the introduction of any fatty (milk, eggs) or alcohol-containing substances. An excellent antidote is also a crude, oxygen-containing turpentine oil (1.0-2.0 gr. Every 1/4 - 1/2 hours, only 5-10 gr.). If the poison has already managed to be absorbed and the collapse begins, then in the foreground agents that stimulate the activity of the heart are shown. At the forensic opening F. suspicious masses (contents of the stomach, intestines, food, drinks, etc.) are distilled, according to Mitcherlich, in a dark room after preliminary acidification with diluted sulfuric acid. In the case of F.'s presence, a characteristic glow is observed at the cooled end of the steam outlet pipe. For the manifestation of the reaction, 1 milligram is enough. F. in 200,000 parts of the liquid. A negative result does not speak, however, against the presence of F., since the presence in the test mass of many substances, such as turpentine oil, chloroform, ether, benzene, chlorine, sulfurous acid, hydrogen sulfide, essential oils, inhibits glow. According to Dussar, the test masses are heated in an apparatus similar to Marshev with pure zinc and sulfuric acid; phosphorous hydrogen released from the flue gas in the flue gas tube burns when ignited in a beautiful emerald green color. The flame is seen in a dark room against a white porcelain plate. This highly sensitive reaction is partly modified, partly masked when some organic volatile substances (hydrogen sulfide, wine alcohol, ether) are present in the test mass, and therefore, according to Blondlo, it is advisable to pass the gas emitted with this method first through a solution of caustic alkali, and then through a solution silver nitrate and the resulting substance (silver phosphorus) are decomposed a second time with zinc and sulfuric acid. Wed Wegner, Der Einfluss des Phosphors auf den Organismus (Arch. Für Pathol. Anatomie und caet., 1872 v. 55, p. 11); Kassowitz, "Die normale Ossification und die Erkrankungen des Knochensystems bei Rachitis und hereditärer Syphilis" (1882); H. Korsakov, “On the Pathogenesis of the English Disease” (dissertation, 1883); Mandelstam, The Physician (1889, Nos. 5, 7, 9, 10 and 11); Shabanova, "The Doctor" (1889, No. 16-19); Busch, "Sitzungsber. Der Niederrheins. Geschichte für Natur und Heilkunde" (1881); Voit, "Zeitschrift für Biologie" (1880, vol. XVI, p. 55); "Eulenhurg" s Real-Encyclop. "(1888, vol. XV, pp. 549 and 554);" Maschk "s" Handbuch "(1888, vol. II, pp. 176-228); Bauer," Der Stoffamsatz bei der Phosphorvergiftung "(" Zeits. für Biologie ", 1871, v. VII, p. 63); Bamberger," Zur Theorie und Behandlung der acuten Phosphorverg. "(" Wirzburg. medicin. Zeitung "(1867); Gager," Manual to a pharmacist and medical surgeon. practice "(1893). See also guidelines on pharmacology (Binz, Rossbach and Notnagel and others) and toxicology (Cobert, Hoffmann and others). M. B. Kotsyn. Phosphorus in living organisms It is part of three organic substances of very important physiological significance: lecithin, nuclein and glycerin-phosphorus. sour In addition, phosphoric acid is found in the body in combination with sodium, potassium, lime and magnesia. The predominance of phosphates in the blood is one of the characteristic features of carnivores, while carbonic compounds predominate in the blood of herbivores, and, like potassium salts, phosphoric acid is found mainly in blood balls, in muscles and in the brain. Finally, the same phosphoric acid in combination with lime makes up the largest part of the inorganic substances that make up bones and teeth. Phosphates are found in all body fluids; but they are especially rich in urine, with which they are excreted from the body, at least in carnivores and in animals with mixed nutrition. Herbivores release phosphates mainly along with intestinal eruptions. In the human nervous system is about 12 grams. phosphoric acid, in the muscular system 130 g., in the bones of the skeleton 1400 g. - F. is secreted from the body in the form of phosphates formed from the decomposition of lecithin, nuclein and glycerol of phosphoric acid and the oxidation of phosphorus-containing products of this cleavage. A person secretes daily from 2.50 to 3.50 grams. phosphoric acid. Most F. is secreted from the body in the form of acid phosphorus. acid salt potassium, which imparts an acid reaction to the urine of carnivores and mixed-fed animals; in addition, due to the same acid salt, phosphates in the urine are in a dissolved state. F. urine refers to all urine nitrogen as approximately 1 to 6 or 7; but this attitude, of course, changes in accordance with the nature of the food. Judging by the fact that F. is a part of such important compounds as lecithin and nuclein and, in addition, it forms an integral part of organs and is predominantly nervous system, muscles and gonads, its value for life should be very outstanding. F. and is listed in a number of nutrients. The formation of acidic phosphates from neutrals is explained by the many effects on these latter organic acids formed during the activity of organs.![]()
- (Latin Phosphorus) P, chemical element V groups periodic system Mendeleev, atomic number 15, atomic mass 30.97376, non-metal. Natural F. consists of one stable isotope 31P; received six artificial radioactive ... ... Great Soviet Encyclopedia
Phosphorus (P) Atomic number 15 Appearance of a simple substance White phosphorus white, waxy, slightly phosphorescent Atom properties Atomic mass (molar mass) 30.973762 a. E. m. (g / mol) The radius of the atom ... Wikipedia
Phosphorus (P) Atomic number 15 Appearance of a simple substance White phosphorus white, waxy, slightly phosphorescent Properties of the atom Atomic mass (molar mass) 30.973762 a. E. m. (g / mol) The radius of the atom ... Wikipedia Wikipedia
- (live silver, Hydrargirum, Quecksilber, mercure), Hg is one of the 7 metals known in antiquity: gold, silver, copper, iron, lead, tin and R. Compared to the other 6 metals, people are most likely ... Encyclopedic Dictionary F.A. Brockhaus and I.A. Efron
Iron - (Ferrum) Metal iron, metal properties, production and use Metal information iron, physical and chemical properties metal, mining and use of iron Content Contents Definition of the term Etymology History of iron Origin ... ... Encyclopedia of the investor
Page 5
Phosphorus exhibits an oxidizing function when interacting with metals: 3Са + 2Р \u003d Са3Р2
As a reducing agent, phosphorus acts in reactions with active non-metals - halogens, oxygen, sulfur, and also with strong oxidizing agents:
2P + 3S \u003d P2S3 2P + 5S \u003d P2S5
It interacts with oxygen and chlorine in a similar way.
P + 5HNO3 \u003d H3PO4 + 5NO2 + H2O
In alkali solutions, when heated, white phosphorus disproportionates:
8Р + 3Ва (ОН) 2 + 6Н2О \u003d 2РН3 + 3Ва (Н2РО2) 2
Chemical phosphorus oxide (+3) has an acidic nature:
P2O3 + 3H2O \u003d 2H3RO3
Phosphorous acid - colorless fusible crystals that are readily soluble in water. By chemical structure it is a distorted tetrahedron, in the center of which there is a phosphorus atom with sp3 - hybrid orbitals, and the vertices are occupied by two hydroxo groups and hydrogen and oxygen atoms. The hydrogen atom, directly connected with phosphorus, is not capable of substitution, and therefore phosphorous acid is a maximum dibasic and is often represented by the formula H2 [HPO3]. Phosphorous acid is an acid of medium strength. Its salts - phosphites are obtained by the interaction of P2O3 with alkalis:
P2O3 + 4NaOH \u003d 2Na2NRO3 + H2O
Phosphites of alkali metals and calcium are readily soluble in water.
When heated, phosphorous acid disproportionates:
4H3RO3 \u003d RN3 + 3H3RO4
Phosphorous acid is oxidized by many oxidizing agents, including halogens, for example:
Н3РО3 + Сl2 + Н2О \u003d Н3РО4 + 2НСl
Usually phosphorous acid is obtained by hydrolysis of phosphorus trihalides:
RG3 + 3H2O \u003d N3RO3 + 3NG
When heating monosubstituted phosphites, salts of pyrophosphorous (diphosphorous) acid - pyrophosphites are obtained:
2NaH2RO3 \u003d Na2H2P2O5 + H2O
Pyrophosphites when boiled with water are hydrolyzed:
Na2H2P2O5 + 3H2O \u003d 2NaOH + 2H3RO3
The pyrophosphorous acid H4P2O5 itself (pentaoxodiphosphoric) itself, as well as phosphorous, is only dibasic and relatively unstable.
Another phosphorus acid (+3) is known - a poorly studied polymeric metaphosphorous acid (HPO2) n.
The most characteristic of phosphorus is P2O5 oxide - diphosphorus pentoxide. It is a white solid that can easily be obtained in a glassy state. In the vaporous state, phosphorus oxide molecules (+5) have the composition P4O10. Solid P2O5 has several modifications. One of the forms of phosphorus oxide (+5) has a molecular structure with P4O10 molecules at the lattice sites. By appearance this modification resembles ice. It has a low density, easily passes into steam, is highly soluble in water and reactive. P2O5 is the strongest dehydrating reagent. The intensity of the draining action is much higher than moisture absorbers such as CaCl2, NaOH, H2SO4 and others. Upon hydration of P2O5, metaphosphoric acid is first formed:
P2O5 + H2O \u003d 2HPO3
further hydration of which sequentially leads to pyrophosphoric and phosphoric acid:
2НРО3 + Н2О \u003d Н4Р2О7 and Н4Р2О7 + Н2О \u003d 2Н3РО4
Phosphoric acid is one of the most important derivatives of phosphorus (+5). These are colorless, fusible, crystals floating in the air, miscible with water in any ratio. In solid acid and concentrated solutions, intermolecular hydrogen bonds act. Therefore, strong H3PO4 solutions are highly viscous. AT aquatic environment phosphoric acid is an acid of medium strength. In an aqueous solution, orthophosphates — salts of phosphoric acid — undergo hydrolysis, and the pH of the medium naturally decreases when passing from a medium to an acid salt.
Na3RO4 + H2O \u003d NaOH + Na2HRO4, pH \u003d 12.1
Na2NRO4 + H2O \u003d NaOH + NaH2RO4, pH \u003d 8.9
During oxidation of wet phosphorus, along with P2O5 and P2O3, phosphoric acid (hexaoxodiphosphoric) acid Н4Р2О6 is formed, in which the oxidation state of phosphorus is +4. In its structure, phosphorus atoms are directly related to each other, unlike polyphosphoric acids:
Н4Р2О6 is an acid of medium strength; all four of its hydrogen atoms can be replaced by a metal. When heating it aqueous solutions acid, adding water, decomposes:
Н4Р2О6 + Н2О \u003d Н3РО3 + Н3РО4
The solutions of its salts - hypophosphates - in water are quite stable. Of the hypophosphates in water, only alkali metal salts are well soluble.
The smallest positive oxidation state of phosphorus in the hypophosphorous (dioxophosphoric) acid H3PO2. It can be obtained in a free state by displacement from salts - hypophosphites, for example:
Ba (H2O2) 2 + H2SO4 \u003d BaSO4 + 2H3RO2
Phosphoric acid - colorless crystals, highly soluble in water. Thus, in hypophosphorous acid, the oxidation state of phosphorus is +1, and its covalency is 5. H3PO2 is a strong acid. This acid and its salts, hypophosphites are the strongest reducing agents.
There are other acids containing phosphorus - mononadphosphoric H3PO5, dinaphosphoric H4P2O8, tetramethaphosphoric (HPO3) 4, pyrophosphoric H4P2O7.
Compounds of phosphorus with non-metals
Phosphorus and hydrogen in the form of simple substances practically do not interact. Hydrogen derivatives of phosphorus are obtained indirectly, for example:
Ca3P2 + 6CHl \u003d 3CaCl2 + 2PH3
Phosphine PH3 is a colorless, highly toxic gas with the smell of rotten fish. The phosphine molecule can be considered as an ammonia molecule. However the angle between n-R-N bonds significantly less than ammonia. This means a decrease in the participation of s-clouds in the formation of hybrid bonds in the case of phosphine. The bonds of phosphorus with hydrogen are less strong than the bonds of nitrogen with hydrogen. The donor properties of phosphine are less pronounced than that of ammonia. The low polarity of the phosphine molecule and the weak activity of accepting the proton lead to the absence of hydrogen bonds not only in the liquid and solid states, but also with water molecules in solutions, as well as to the low resistance of the phosphonium ion PH4 +. The most stable in the solid state salt of phosphonium is its iodide PH4I. Water and especially alkaline solutions of phosphonium salts decompose vigorously:
PH4I + KOH \u003d PH3 + KI + H2O
Phosphine and phosphonium salts are powerful reducing agents. In air, phosphine burns to phosphoric acid:
RN3 + 2O2 \u003d H3RO4
During the decomposition of active metal phosphides by acids simultaneously with phosphine, diphosphine P2H4 is formed as an impurity. Diphosphine is a colorless volatile liquid, similar in structure to hydrazine, but phosphine does not exhibit basic properties. It self-ignites in the air, decomposes when stored in the light and when heated. Phosphorus, phosphine and a yellow amorphous substance are present in its decay products. This product is called solid phosphorous hydrogen and is assigned the formula P12H6.
Phosphorus forms tri- and pentahalides with halogens. These phosphorus derivatives are known for all analogues, but chlorine compounds are practically important. WG3 and WG5 are toxic, obtained directly from simple substances.
RG3 - stable exothermic compounds; PF3 is a colorless gas, PCl3 and PBr3 are colorless liquids, and PI3 are red crystals. In the solid state, all trihalides form crystals with a molecular structure. RG3 and RG5 are acid-forming compounds:
PI3 + 3H2O \u003d 3HI + H3PO3
Both phosphorus nitrides are known, which correspond to three- and five-covalent states: PN and P2N5. In both compounds, nitrogen is trivalent. Both nitrides are chemically inert, resistant to water, acids and alkalis.
Melted phosphorus dissolves sulfur well, but chemical interaction occurs at high temperature. Of phosphorus sulfides, P4S3, P4S7, P4S10 are better studied. These sulfides can be recrystallized in a naphthalene melt and precipitated as yellow crystals. When heated, sulfides ignite and burn with the formation of P2O5 and SO2. With water, they all slowly decompose with the release of hydrogen sulfide and the formation of oxygen acids of phosphorus.
Compounds of phosphorus with metals
With active metals, phosphorus forms salt-like phosphides that obey the rules of classical valency. p-metals, as well as metals of the zinc subgroup give both normal and anionic phosphides. Most of these compounds exhibit semiconductor properties, i.e. the dominant bond in them is covalent. The difference between nitrogen and phosphorus, due to dimensional and energy factors, is most characteristic for the interaction of these elements with transition metals. For nitrogen, when interacting with the latter, the main thing is the formation of metal-like nitrides. Phosphorus also forms metal-like phosphides. Many phosphides, especially with predominantly covalent bondrefractory. So, AlP melts at 2197 degrees C. and gallium phosphide has a melting point of 1577 degrees C. Phosphides of alkali and alkaline earth metals are easily decomposed by water with the release of phosphine. Many phosphides are not only semiconductors (AlP, GaP, InP), but also ferromagnets, such as CoP and Fe3P.


