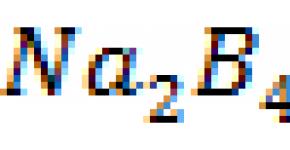Phosphorus Oxide Phosphoric Acid Salt. Phosphorus Oxides and Phosphoric Acids
The phosphorus element forms a number of oxides, the most important of which are phosphorus (III) oxide. P2O3and phosphorus oxide (V) P2O5 .
Phosphorus (III) oxide, or phosphorous anhydride (P2O3)obtained by slow oxidation of phosphorus, burning it in a lack of oxygen. It is a waxy crystalline white mass with a melting point of 22.5 ° C. Toxic.
Chemical properties:
1) reacts with cold water, forming phosphorous acid H3PO3;
2) interacting with alkalis, forms salts - phosphites;
3) is a strong reducing agent.
Interacting with oxygen, it is oxidized to phosphorus oxide (V) P2O5.
Phosphorus (V) Oxide, or Phosphoric Anhydride (P2O5)obtained by burning phosphorus in air or in oxygen. It is a white crystalline powder, with a melting point of 36 ° C.
Chemical properties:
1) interacting with water, forms orthophosphoric acid H3PO4;
2) having the properties of an acid oxide, it reacts with basic oxides and hydroxides;
3) capable of absorbing water vapor.
End of work -
This topic belongs to the section:
Inorganic Cheat Sheet
Cheat sheet for inorganic chemistry ... Olga Vladimirovna Makarova ...
If you need additional material on this topic, or you did not find what you were looking for, we recommend using the search on our database of works:
What we will do with the material received:
If this material turned out to be useful for you, you can save it to your page in social networks:
| Tweet |
All topics in this section:
Matter and its motion
Matter is an objective reality with the property of motion. Everything that exists is different kinds of moving matter. Matter exists independently of consciousness.
Substances and their change. Inorganic chemistry subject
Substances - types of matter, discrete particles of which have a finite rest mass (sulfur, oxygen, lime, etc.). Physical bodies are made up of substances. Every
Periodic system of elements D.I. Mendeleev
The periodic law was discovered in 1869 by D.I. Mendeleev. He also created a classification of chemical elements, expressed in the form of a periodic system. To me
The value of the periodic system of Mendeleev.
The periodic system of elements was the first natural classification of chemical elements, which showed that they are interconnected with each other, and also served as a further study.
Theory of chemical structure
The theory of chemical structure was developed by A.M. Butlerov. It has the following positions: 1) the atoms in the molecules are connected to each other
General characteristics of P-, S-, D-elements
Elements in the periodic table are divided into s-, p-, d-elements. This division is based on how many levels the electron shell of an atom of an element has.
Covalent bond. Valence bond method
A chemical bond made by common electron pairs arising in the shells of bound atoms having antiparallel spins is called atomic, or covalent
Nonpolar and polar covalent bonds
Using a chemical bond, the atoms of the elements in the composition of the substances are held together. The type of chemical bond depends on the distribution of electron density in the molecule.
Multicenter Communications
During the development of the valence bond method, it turned out that the real properties of the molecule are intermediate between those described by the corresponding formula. Such molecules
Ionic bond
A bond arising between atoms with sharply expressed opposite properties (typical metal and typical non-metal), between which electrostatic attraction forces arise
Hydrogen bond
In the 80s of the XIX century. M.A. Ilyinsky N.N. Beketov found that a hydrogen atom connected to an atom of fluorine, oxygen, or nitrogen can form
Chemical conversion of energy
A chemical reaction is the conversion of one or more of the starting materials into others by the chemical composition or structure of the substance. Compared to nuclear reactors
Chain reactions
There are chemical reactions in which the interaction between the components is quite simple. There is a very extensive group of reactions that proceed complexly. In these reactions
General properties of non-metals
Based on the position of non-metals in the periodic system of Mendeleev, it is possible to identify properties characteristic of them. You can determine the number of electrons on the external
Hydrogen
Hydrogen (H) - the 1st element of the periodic system of Mendeleev - I and VII group, the main subgroup, 1 period. On the external s1 sublevel, there is 1 valence electron and 1 s2
Hydrogen peroxide
Peroxide, or hydrogen peroxide - an oxygen compound of hydrogen (peroxide). Formula: Н2О2 Physical properties: hydrogen peroxide - colorless syrup
General characteristics of the halogen subgroup
Halogens - elements of group VII - fluorine, chlorine, bromine, iodine, astatine (astatine is poorly studied in connection with its radioactivity). Halogens are pronounced non-metals. Only iodine in re
Chlorine. Hydrogen chloride and hydrochloric acid
Chlorine (Cl) –stands in the 3rd period, in the VII group of the main subgroup of the periodic system, serial number 17, atomic mass 35.453; refers to halogens.
Brief information about fluoride, bromine and iodine
Fluorine (F); bromine (Br); iodine (I) belong to the group of halogens. They stand in the 7th group of the main subgroup of the periodic system. General electronic formula: ns2np6.
General characteristics of the oxygen subgroup
A subgroup of oxygen, or chalcogenes - the 6th group of the periodic system D.I. Mendeleva, including the following elements: 1) oxygen - O; 2) sulfur
Oxygen and its properties
Oxygen (O) is in period 1, group VI, in the main subgroup. p-element. Electronic configuration 1s22s22p4. The number of electrons on the outer ur
Ozone and its properties
In the solid state, oxygen has three modifications:? -,? - and? - modifications. Ozone (O3) —one of the allotropic modifications of oxygen
Sulfur and its properties
Sulfur (S) in nature is found in compounds and in free form. Sulfur compounds such as PbS lead gloss, ZnS zinc blende, Cu copper gloss are also common.
Hydrogen sulfide and sulfides
Hydrogen sulfide (H2S) is a colorless gas with a pungent smell of rotting protein. In nature, there are inputs of mineral keys of volcanic gases, rotting garbage, and also
Properties of sulfuric acid and its practical significance
The structure of the sulfuric acid formula: Preparation: the main method for the production of sulfuric acid from SO3 is the contact method.
Chemical properties.
1. Concentrated sulfuric acid is a strong oxidizing agent. Redox reactions require heating, and the reaction product is mainly SO2.
Receiving.
1. In industry, nitrogen is obtained by liquefying air, followed by evaporation and separation of nitrogen from other gas fractions of the air. The resulting nitrogen contains impurities of noble gases (argon).
General characteristics of the nitrogen subgroup
Subgroup of nitrogen - the fifth group, the main subgroup of the periodic system D.I. Mendeleev. It includes elements: nitrogen (N); phosphorus (P); arsenic (
Ammonium chloride (nitrogen chloride).
Obtaining: in the industry until the end of the Х1Х centuries, ammonia was obtained as a by-product in the coking of coal, which contains up to 1-2% nitrogen. At the beginning
Ammonium salts
Ammonium salts are complex substances, including ammonium cations NH4 + and acid residues. Physical properties: ammonium salts - t
Nitrogen oxides
With oxygen N forms oxides: N2O, NO, N2O3 NO2, N2O5 and NO3. Nitric oxide I - N2O - nitrous oxide, "laughing gas". Physical properties:
Nitric acid
Nitric acid is a colorless, “smoking” liquid in the air with a pungent odor. The chemical formula is HNO3. Physical properties. At temperature
Allotropic modifications of phosphorus
Phosphorus forms several allotropic modifications - modifications. The phenomenon of allotropic modifications in phosphorus is caused by the formation of various crystalline forms. White phospho
Phosphoric acid.
Phosphoric anhydride corresponds to several acids. The main one is orthophosphoric acid H3PO4. Dehydrated phosphoric acid is presented as colorless transparent crystals.
Mineral fertilizers
Mineral fertilizers - inorganic substances, mainly salts, which include nutrients necessary for plants and are used to increase fertility
Carbon and its properties
Carbon (C) - typical non-metal; in the periodic system is in the 2nd period of group IV, the main subgroup. Sequence number 6, Ar \u003d 12.011 amu, nuclear charge +6.
Allotropic carbon modifications
Carbon forms 5 allotropic modifications: cubic diamond, hexagonal diamond, graphite and two forms of carbine. Hexagonal diamond found in meteorites (mineral
Carbon oxides. carbonic acid
Carbon with oxygen forms the oxides: СО, СО2, С3О2, С5О2, С6О9, etc. Carbon monoxide (II) - СО. Physical properties: carbon monoxide, b
Silicon and its properties
Silicon (Si) is in period 3, group IV of the main subgroup of the periodic system. Physical properties: silicon exists in two versions: amo
There are three types of internal structure of primary particles.
1. Suspenzoids (or irreversible colloids) are heterogeneous systems whose properties can be determined by a developed interphase surface. In comparison with suspensions more finely dispersed
Silicic acid salts
The general formula of silicic acids is n SiO2? M H2O. In nature, they are mainly in the form of salts, few are isolated in free form, for example, HSiO (ortho
Obtaining cement and ceramics
Cement is the most important material in construction. Cement is obtained by roasting a mixture of clay with limestone. When firing a mixture of CaCO3 (soda ash)
Physical properties of metals
All metals have a number of common, characteristic properties. Common properties are: high electrical conductivity and thermal conductivity, ductility. The scatter of parameters for met
Chemical properties of metals
Metals possess a low ionization potential and electron affinity; therefore, they act as reducing agents in chemical reactions and form in solutions
Metals and alloys in engineering
In the periodic system of 110 known elements, 88 are metals. In the 20th century, using nuclear reactions, radioactive metals that did not exist
The main methods for producing metals
A large number of metals are found in nature in the form of compounds. Native metals are those that are found in a free state (gold, platinum, p
Metal corrosion
Corrosion of metals (corrosio - corrosion) is the physicochemical reaction of metals and alloys with the environment, as a result of which they lose their properties. At the heart of
Protection of metals from corrosion
Protection of metals and alloys from corrosion in aggressive environments is based on: 1) increasing the corrosion resistance of the material itself; 2) decrease in aggressiveness
General characteristics of the lithium subgroup
Lithium subgroup - 1 group, the main subgroup - includes alkali metals: Li - lithium, Na - sodium, K - potassium, Cs - cesium, Rb - rubidium, Fr - France. Common electron
Sodium and potassium
Sodium and potassium are alkaline metals, are in the 1st group of the main subgroup. Physical properties: similar in physical properties: light silver
Caustic alkali
Alkalis form alkali metal hydroxides of the 1st group of the main subgroup when dissolved in water. Physical properties: alkaline solutions in water
Sodium and potassium salts
Sodium and potassium form salts with all acids. The sodium and potassium salts are very similar in chemical properties. A characteristic feature of these salts is their good solubility in water, therefore
General characteristics of the beryllium subgroup
The beryllium subgroup includes: beryllium and alkaline earth metals: magnesium, strontium, barium, calcium and radium. The most common in nature in the form of compounds,
Calcium
Calcium (Ca), a chemical element of the 2nd group of the periodic system, is an alkaline earth element. Natural calcium consists of six stable isotopes. Conf
Calcium Oxide and Hydroxide
Calcium oxide (CaO) - quicklime or burnt lime - a white fire-resistant substance formed by crystals. Crystallizes in a cubic face-centered crystal
Water hardness and methods for its elimination
Since calcium is widely distributed in nature, its salts are found in large quantities in natural waters. Water, which has in its composition salts of magnesium and calcium, is called
General characteristics of the boron subgroup
The external electronic configuration for all elements of the subgroup is s2p1. A characteristic property of subgroup IIIA is the complete absence of metallic properties in boron and ty
Aluminum. The use of aluminum and its alloys
Aluminum is located in the 3rd group of the main subgroup, in the 3rd period. Sequence number 13. Atomic mass ~ 27. P-element. Electronic configuration: 1s22s22p63s23p1. Outside
Aluminum oxide and hydroxide
Alumina - Al2O3. Physical properties: alumina is a white amorphous powder or very hard white crystals. Molecular mass \u003d 101.96, density - 3.97
General characteristics of the chromium subgroup
Elements of the subgroup chromase occupy an intermediate position in the series of transition metals. They have high melting and boiling points, free space on electronic
Oxides and hydroxides of chromium
Chromium forms three oxides: CrO, Cr2O3 and CrO3. Chromium oxide II (CrО) - basic oxide - black powder. Strong reducing agent. CrO dissolves in dilute hydrochloric acid
Chromates and Dichromates
Chromates are salts of chromic acid Н2Сг04, which exists only in aqueous solutions with a concentration of no higher than 75%. Chromate valence in chromates - 6. Chromates
General characteristics of the iron family
The iron family is part of the side subgroup of the eighth group and is the first triad in it, including iron, cobalt nickel
Iron compounds
Iron (II) oxide FeO– a black crystalline substance, insoluble in water and alkalis. FeO corresponds to the base Fe (OH) 2.
Domain process
Blast furnace process - smelting of cast iron in a blast furnace. The blast furnace is laid out with refractory bricks 30 m high and an internal diameter of 12 m. The upper half - w
Cast Iron and Steel
Iron alloys are metal systems whose main component is iron. Classification of iron alloys: 1) alloys of iron with carbon (n
Heavy water
Heavy water - deuterium oxide D2O with oxygen of natural isotopic composition, a colorless liquid, odorless and tasteless. Heavy water was open
Chemical and physical properties.
Heavy water has a boiling point of 101.44 ° C, and a melting point of 3.823 ° C. D2O crystals have the same structure as ordinary ice crystals, the difference in size
Hydrochloric acid salts
Salts of hydrochloric acid or chlorides are chlorine compounds with all elements having a lower value of electronegativity. Metal chlorides
Oxides and acids of phosphorus.
Chemistry of boron.
Electronic configuration:
Boron is relatively rare in nature. TO major natural compounds boron include boric acid and salts of boric acids (the most known borax ![]() ).
).
Boron is located in the third group of the periodic system, but according to its properties most similarnot with other elements of this group, but with an element of the fourth group - silicon (manifestation of "diagonal similarity").
Like silicon, boron forms compounds with metals, many of which are characterized by high hardness and high melting points.
Free boron get reduction of boric anhydride with magnesium (boron is released as an amorphous powder contaminated with impurities).
Pure crystalline boron get thermal decomposition or reduction of its halides, as well as the decomposition of hydrogen boron compounds. It has a black color and, among simple substances, is second only to diamond in hardness.
Water does not affect boron; concentrated sulfuric and nitric acids oxidize it into boric acid. For example:
B + 3HN \u003d + 3N
At room temperature, boron combines only with fluorine; it does not oxidize in air.
If the amorphous boron is heated to 700 ° C, then it lights up and burns with a reddish flame, turning into oxide; a large amount of heat is released:
4V (K) + 30 2 (g) \u003d 2V 2 0 3 (k), ∆Н \u003d -2508 kJ.
At high temperature boron combines with many metals, forming borides, for example, magnesium boride Mg 3 B 2.
Many borides are very solid and chemically stable, and retain these properties at high temperatures. They are also characterized by refractoriness.
With halogensboron also reacts when heated and forms substances of the general formula VG 3. In these compounds, boron forms flat molecules with halogens with angles between the bonds H-B-D equal to 120 °. Such a geometry of molecules is expected when considering the repulsion of electron pairs of the valence shell and based on the bp2 hybridization of boron orbitals.
Boron halides, like other boron compounds of non-polymer structure, are electron-deficient. So, in a boron trifluoride molecule with a linear combination of 2s, 2px, 2pu, 2rg AO of boron and one valence p-AO (with an unpaired electron) of each fluorine atom, 4 + 3 \u003d 7 molecular orbitals are formed. On these MOs, 3 + 3 \u003d 6 electrons are arranged in pairs. Thus, in the boron trifluoride molecule, 7 - 6/2 \u003d 4 MO remain unoccupied. One of the MOs, perpendicular to the plane of the molecule, is not involved in binding to fluorine atoms. But it has a rather low energy due to relaxation (compression in this case, see Fig. 4.35 and 4.36) of the boron p-orbitals under the action of strongly electronegative fluorine. Therefore, the placement of an electron pair of another atom or ion on a given MO becomes energetically favorable, and the BF3 molecule can, therefore, be an acceptor of an electron pair. Indeed, BF3 combines in a donor-acceptor way with water, ammonia and other substances; The complex anion BF4 is also known. Formally, this process can be represented by the scheme:

In all such compounds, the covalence and coordination number of boron are four, and the boron atom forms tetrahedral structures, which are determined by the minimum energy. This is explained by the repulsion of 4 electron pairs of the valence shell or sp3 hybridization of the boron atom.
Hydrogen borodes (boranes). Under the action of hydrochloric acid on magnesium boride Mg3B2, a complex mixture of various borohydrogen is obtained. The following borohydrogen was isolated in pure form from this mixture:
The main product of the interaction of magnesium boride with hydrochloric acid is tetraborane B 4 H 10 - a volatile liquid (temp. Boiling point 18 ° C), the vapor of which ignites in air. During storage, the tetraborane gradually decomposes with the formation of the simplest of the obtained borohydrates - diborane B\u003e H6. The latter is a gas condensing into a liquid at -92.5 ° C. In air, it does not catch fire, but with water, like other borohydrogen, it decomposes immediately with the removal of hydrogen and the formation of boric acid H 3 BO 3:
B 2 H 6 + 6 H 2 0 - 2 H 3 BO 3 + 6 H 2.
Boron atoms in boron hydrogen molecules are connected to each other by hydrogen "bridges", for example:
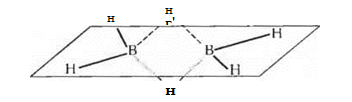
The dashed line and the dashed line in this diagram show three-center bonds: here the total pair of electrons occupies a molecular orbital that spans three atoms — the “bridge” hydrogen atom and both boron atoms. Such an orbital is formed due to the overlap of the ls-orbital of the hydrogen atom with the sp 3 -hybrid orbitals of two boron atoms. Four “terminal” hydrogen atoms are bonded to boron atoms by the usual two-center two-electron bonds. Thus, of the twelve valence electrons present in the atoms that make up the diborane molecule, eight participate in the formation of two-center B-H bonds, and four form two three-center B-H-B bonds.
Boron oxide, or boric anhydride, B 2 0 3 can be obtained either by directly combining boron with oxygen or by calcining boric acid. Boric anhydride is very flame retardant and does not recover with coal, even with white heat. In water, it dissolves with the formation of orthoboric acid and the release of heat:
Br0 3 (k) + 3H 2 0 (x) \u003d 2HzB0 3 (p),
DN \u003d -76.5 kJ.
Orthoboric acid H 3 VO 3 is a white crystals, the shiny scales of which are dissolved in hot water. In an aqueous solution, orthoboric acid is in equilibrium with other boron acids:
As a rule, of the various acids of the same element, which differ from each other in the degree of hydration (i.e., the number of bound water molecules), the most hydrated form is the most stable in aqueous solutions. Of the above boron acids, orthoboric acid is the most stable in aqueous solutions. Therefore, in cases where during the reaction some boric acid is to be obtained, its most stable form, orthoboric acid, is always released in aqueous solutions. When the solution is cooled, boric acid crystallizes out, since it is poorly soluble in cold water.
Orthoboric acid is one of the very weak acids. Its less hydrated forms (HBO 2, Н 2 В 4 О 7) are also weak acids, but somewhat stronger than orthoboric acid. Such a rule about the greater strength of less hydrated forms of the same acid holds in other cases. Therefore, of weak boric acids, tetraboric acid is somewhat stronger.
As a result, when trying to neutralize an aqueous solution of orthoboric acid with alkali, it is not orthoborate, but tetraborate of the alkaline element:
4H 3 B0 3 + 2NaOH \u003d Na 2 B 4 0 7 + 7H z O.
For the most part, salts of boric acids borates are not derivatives of orthoboric acid Н3ВО3, but tetraboric and other boric acids, which are poorer in water.
Compounds of boron with nitrogen have two polymorphic modifications: diamond-like and graphite-like. The graphite-like modification of boron nitride has a graphite structure in which boron atoms alternate with nitrogen atoms, both in planes formed by six-membered rings and in planes perpendicular to the layers.
Ticket 2.
2. Oxides and their hydrated forms of group V elements.
The main subgroup of group V of the periodic system includes nitrogen, phosphorus, arsenic, antimony, and bismuth.
These elements, having five electrons on the outer electron shell of an atom, are generally characterized as non-metals. Due to the presence of five external electrons, the highest positive oxidation state of the elements of this subgroup is -1-5, and the negative one -3.
Nitrogen oxides.
Nitrogen forms a series of oxides with oxygen; all of them can be obtained from nitric acid or its salts.
Nitric oxide (I), or nitrous oxide, N 2 O is obtained by heating ammonium nitrate:
NH 4 NO 3 \u003d N 2 0 + 2 H 2 0.
Nitric oxide (I) is a colorless gas that is used as anesthesia.
Nitric oxide (I) is a thermodynamically unstable compound. Gibbs standard energy of its formation is positive. However, due to the high strength of the bonds in the N 2 0 molecule, the activation energies of the reactions proceeding with the participation of this substance are high. In particular, the activation energy of N 2 0 decay is high. Therefore, nitric oxide (I) is stable at room temperature. However, at elevated temperatures, it decomposes into nitrogen and oxygen; the decomposition is faster, the higher the temperature.
Nitric oxide (I) does not react with water, nor with acids, or with alkali.
Nitric oxide (II ), or nitric oxide, N0 is a colorless, difficult to liquefy gas.
By its chemical properties, nitric oxide (II) is among the indifferent oxides, since it does not form any acid.
Like N20, the nitrogen oxide (II) is thermodynamically unstable. At room temperature, NO does not decompose, because its molecules are strong enough. Only at temperatures above 1000 ° C does its decomposition into nitrogen and oxygen begin to flow at a noticeable rate.
In the laboratory, nitric oxide (II) is usually obtained by the interaction of 30-35% nitric acid with copper:
3Cu + 8HNO 3 \u003d 3Cu (N0 3) 2 + 2NOt + 4H 2 0.
In industry, it is an intermediate in the production of nitric acid.
Nitric oxide (II) is characterized by redox duality. Under the influence of strong oxidizing agents, it is oxidized, and in the presence of strong reducing agents it is restored. For example, it is easily oxidized by atmospheric oxygen to nitrogen dioxide:
2NO + 0 2 \u003d 2N0 2.
At the same time, a mixture of equal volumes of N0 and H 2 explodes when heated: 2NO (r) + 2H 2 (G) \u003d N 2 (r) 4-2H 2 0 (G), AN \u003d -655 kJ.
Dioxide (or dioxide) of nitrogen N0 2 - brown poisonous gas. A change in the color of nitrogen dioxide with increasing temperature is accompanied by a change in its molecular weight.
Nitrogen dioxide is a very energetic oxidizing agent. Many substances can burn in an atmosphere of NO 2, taking oxygen from it.
When dissolved in water, NO 2 reacts with water to form nitric and nitrous acids: 2N0 2 + H 2 0 \u003d HNO 3 + HNO 2
Therefore, in practice, the interaction of nitrogen dioxide with water, especially with hot, proceeds according to the equation 6N0 2 + 2H 2 0 \u003d 4HN0 3 + 2N0, which can be obtained by adding the two previous equations, if previously the first of them is multiplied by three.
In the presence of air, the resulting nitric oxide is immediately oxidized to nitrogen dioxide, so that in this case, NO 2 ultimately passes into nitric acid:
4N0 2 + 0 2 + 2H 2 0 \u003d 4HN0 3
If nitrogen dioxide is dissolved in alkalis, then a mixture of salts of nitric and nitrous acids is formed, for example: 2N0 2 + 2NaOH \u003d NaN0 3 + NaN0 2 + H 2 0.
Nitric oxide (III) or nitrous anhydride, N 2 O 3 is a dark blue liquid, decomposing even at low temperatures into N0 and NO 2. A mixture of equal volumes of N0 and NO 2 upon cooling forms N 2 O 3 again: NO + NO 2<=* N 2 O 3
Nitric oxide corresponds to nitrous acid HNO 2.
Nitric oxide (V), or nitric anhydride, N 2 O 5 - white crystals, gradually decomposing even at room temperature into NO 2 and О 2 - It can be obtained by the action of phosphoric anhydride on nitric acid: 2HNO 3 + P 2 O 5 - N 2 O 5 + 2HPO 3
Nitric oxide (V) is a very strong oxidizing agent. In water, nitric oxide (V) dissolves well with the formation of nitric acid.
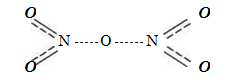 In solid state.
In solid state.
Acids:
Nitrous acid.
When a nitrite diluted with sulfuric acid acts on a solution of a nitrite, free nitrous acid is obtained:
2NaN0 2 + H 2 S0 4 \u003d Na 2 S0 4 + 2HN0 2.
It is one of the weakest and is known only in highly dilute aqueous solutions. When the solution is concentrated or when heated, nitrous acid decomposes:
2HN0 2 \u003d N0 + N0 2 + H 2 0.
HN0 2 exhibits redox duality. Under the action of reducing agents, it is reduced (usually to N0), and in reactions with oxidizing agents it is oxidized to HNO3. The following reactions are examples:
2HN0 2 + 2KI + H 2 S0 4 \u003d 2N0 + I 2 + K 2 S0 4 + 2H 2 0:
5HN0 2 + 2KMn0 4 + 3H 2 S0 4 \u003d 5HN0 3 + 2MnS0 4 + K 2 S0 4 + 3H 2 0.
Nitric acid.
Read ticket number 4.
Oxides and acids of phosphorus.
The most important phosphorus oxides are P 2 0 3 and P 2 0 5.
Phosphorus (III) oxide or phosphorous anhydride, P 2 O 3 is obtained by slow oxidation of phosphorus or when phosphorus burns out with insufficient oxygen supply. Its molecular mass at low temperatures corresponds to the formula P 4 0 6. Under the action of cold water, phosphorus (III) oxide slowly interacts with it, forming phosphorous acid H 3 PO 3. Both phosphorus (III) oxide and phosphorous acid have strongly expressed reducing properties.
Phosphorus Oxide (V), or phosphoric anhydride, P 2 0 5 is formed during the combustion of phosphorus in air or in oxygen in the form of a white voluminous snowy mass.
Phosphorus Oxide (V) greedily combines with water and is therefore used as a very strong dewatering agent. In air, phosphorus oxide (V), attracting moisture, quickly turns into a spreading mass of metaphosphoric acid.
Phosphoric acid.
Phosphorus oxide (V) meets several acids. The most important of them is orthophosphoric acid НзР0 4, usually called simply phosphoric acid. Other phosphoric acids are polymeric compounds. In the anion of all phosphoric acids, a phosphorus atom in the state of “sp 3 hybridization” is surrounded by four oxygen atoms located at the vertices of the tetrahedron. Phosphoric acid is built from isolated tetrahedra; in other phosphoric acids, the PO 4 tetrahedra are combined through oxygen atoms into aggregates containing from two to a very large number - about 10 5 - of phosphorus atoms.
Phosphoric acid is not a strong acid. Being a tribasic, it forms three rows of salts: medium and acid with one or two hydrogen atoms in the acid residue. Medium salts of phosphoric acid are called orthophosphates or simply phosphates, acidic - hydrophosphates:
In the laboratory, phosphoric acid can be obtained by oxidizing phosphorus with 30% HNO 3. The reaction proceeds according to the equation:
ЗР + 5HN0 3 + 2Н 2 0 \u003d ЗН 3 РО 4 + 5N0
In industry, phosphoric acid is obtained by two methods: extraction and thermal. The extraction method is based on the processing of natural phosphates with sulfuric acid:
Ca 3 (P0 4) 2 + 3H 2 S0 4 \u003d 3 CaS0 4 + 2H 3 P0 4
All other phosphoric acids are products of the compound of P04 tetrahedra. Most of these acids are not isolated in the free state, but are known as mixtures, in aqueous solutions or in the form of salts.
Phosphorus oxides. Phosphoric anhydride P; 05 (the "simplest" formula) is the most stable phosphorus oxide under ordinary conditions. This is a white crystalline (or vitreous) very hygroscopic substance of the composition P4O10. Each phosphorus atom is surrounded by four oxygen atoms: P4O, 0 actively interacts with water and also takes it away from other compounds, forming, depending on the conditions, either metaphosphoric HPOj or orthophosphoric HjPO * or pyrophosphoric Н4Р2О7 acid [see reactions (12.5)]. That is why RFO | 0 is widely used as a desiccant of various substances from water vapor. Phosphorous anhydride is described by the simplest formula P203 and the true formula P4Ob: Phosphorus in P4Ob is coordinatively unsaturated and therefore unstable. Phosphorus (III) dioxide is a white waxy mass formed during the oxidation of phosphorus under conditions of oxygen deficiency [see reaction equation (16.4)]. The interaction of P06 with water leads to the formation of phosphorous acid P406 + 6H20 \u003d 4H3P03 Gaseous HC1 decomposes P4Ob: Phosphoric acids - under this name, acids containing phosphorus atoms in the oxidation state +5 are combined. Of the three phosphoric acids, the orthophosphoric acid HjP04 (often called simply phosphoric acid) —white solid \u003d 44.4 ° C), which is highly soluble in water, has the greatest practical value. In an aqueous solution, it dissociates stepwise, forming three types of anions (dihydrophosphates Н2Р04 ~, hydrophosphates НР042 ~ and phosphates Р043 ~) and being in equilibrium with them in accordance with the dissociation constants: The diagram in Fig. 1 illustrates this very clearly. 16.2, showing the proportion of each of the particles depending on the acidity of the solution (pH of the solution). For example, the fraction of acid itself a (H3P04) defined by the formula prevails if. Conversely, only at pH\u003e p £ s does the proportion of phosphate ions P043 ~ 1 begin to prevail. Intermediate ions dominate at pH values \u200b\u200bthat are between pK2 and pKb. All dihydrogen phosphates are soluble in water. Of the hydrophosphates and phosphates in water, only alkali metal and ammonium salts are soluble (see solubility table). Salts of phosphoric acid are valuable mineral fertilizers. The most common among them are superphosphate, precipitate and phosphate rock. Simple superphosphate is a mixture of calcium dihydrogen phosphate Ca (H2P04) 2 and “ballast” CaS04. It is obtained by treating phosphorites and apatites with sulfuric acid. When processing mineral phosphates with phosphoric acid, the product is obtained. 16.2. The proportion of equilibrium gastric species depending on the pH of the solution during multistage dissociation of Н3Р04 yields double superphosphate Ca (Н2Р04) 2. When quenching phosphoric acid with lime, a precipitate is obtained. Complex fertilizers (i.e., containing both nitrogen and phosphorus; or nitrogen, phosphorus and potassium) are important. Of these, ammophos is the most useful - a mixture of NHJH2P04 and (NH ^ HPCV As well as orthophosphoric acid, pyrophosphoric Н4Р2О7 (tetrabasic) is an acid of medium strength. The salts of this acid - pyrophosphates - hydrolyze in aqueous solutions and give a slightly alkaline medium. Pyrophosphoric acid is obtained either by heating 100% phosphoric acid, or adding phosphorus dioxide (V) to the latter: Unlike other phosphoric acids, metaphosphoric НР03 - a strong acid and, as a result, its salts - metaphosphates - are not hydrolyzed by water. When water is absorbed by phosphorus (V) dioxide: By “taking” water from 100% non HN03, phosphorus (V) dioxide also forms metaphosphoric acid: When boiling a solution of metaphosphoric acid, orthophosphoric acid is formed: Lower phosphoric acids. It was shown above that during the interaction of P406 phosphorous acid HjP03 is formed with water: Despite the fact that the acid contains three hydrogen atoms, it is dibasic, since the third hydrogen atom is not acidic, that is, it does not dissociate in aqueous solutions and is not replaced (see structure). Phosphorous acid - an acid of medium strength forms two rows of salts - phosphites or hydrophosphites. Also known is the monobasic phosphoric acid H3PO2 of medium strength (I0 ~ 2) f salts - hypo-phosphites.