Phosphorus and its compounds
The Paris Library contains a manuscript on alchemy, which describes phosphorus discovery. If you believe the document, Alhid Bahil managed to isolate the element in its pure form for the first time.
He lived in the 12th century. Phosphorusthe man received, distilling urine with lime and. The alchemist called the luminous substance escarbukle. Modern name the element was given by Henning Brand.
He combined the Greek words "light" and "bear." German highlighted white phosphorusin 1669, having documented his merit, speaking to the scientific community.
Henning Brand, like Alhid Bachil, used evaporated urine, but heated it with white sand. In the 17th century, and in the 12th, the glow of the resulting substance seemed a miracle. From contemporaries to physical phosphorus properties a different look.
Physical and chemical properties of phosphorus
Phosphorus elementglows due to oxidation processes. Interaction with oxygen passes quickly, self-ignition is possible.
The rapid and abundant release of chemical energy leads to its transition into the energy of light. The process takes place even at room temperature.
That’s the secret of shining phosphorus. Oxygenthe easiest way to react with a white modification of an element. It can be confused with wax, candle wax. The substance melts already at 44 degrees Celsius.
Phosphorus propertieswhite color differ from the properties of other modifications of the element. They, for example, are not toxic.
Colorless phosphorus is poisonous, insoluble in water. She, as a rule, and block the oxidation of the powder. Without reacting with water, white phosphoruseasily soluble in organics, for example, carbon disulfide.
In the first modification phosphorus substanceleast dense. There are only 1,800 grams per cubic meter. At the same time, the lethal dose for humans is only 0.1 grams.
More poisonous yellow phosphorus. In fact, this is a kind of white, but not peeled. The density of the substance is the same, flammability, too.

The melting point is slightly lower - 34 degrees. Boils the element at 280 degrees Celsius. Due to pollution, burning produces thick smoke. Yellow phosphorus, like white, does not react with water.
There is still red phosphorus. It was first received in the 1847th year. The Austrian chemist Schreetter heated the white modification of the element to 500-degrees in an atmosphere of carbon monoxide.
The reaction was carried out in a sealed flask. The resulting type of phosphorus turned out to be thermodynamically stable. A substance is dissolved, perhaps, in some molten metals.
Ignite phosphorus atomcan only when the atmosphere warms up to 250 degrees Celsius. An alternative is active friction, or a strong blow.
The color of red phosphorus is not only red, but also purple. There is no glow. Almost no toxicity. The toxic effect of the red modification of the element is minimal. Therefore, it is scarlet phosphorus that is widely used in industry.
The penultimate modification of an element is black. Received in the 1914th year, is the most stable. The substance has a metallic luster. The surface of black phosphorus is glossy, similar to.
Modification is not amenable to any solvent; it ignites only in an atmosphere heated to 400 degrees. Phosphorus mass black is greatest, as is density. The substance is "born" from white at a pressure of 13,000 atmospheres.

If you bring the pressure to ultrahigh, the last, metal modification of the element appears. Its density reaches almost 4 grams per cubic centimeter. Phosphorus formuladoes not change, but the crystal lattice is transformed. It becomes cubic. The substance begins to conduct electric current.
Phosphorus
Phosphorus oxideserves as a smoke generating agent. Flammable, the yellow modification of the element gives a thick veil that comes in handy in the defense industry.
In particular, phosphorus is added to the tracer bullet. Leaving behind a smoky trail, they allow you to adjust the direction, accuracy of the sends. The “track” is maintained for a kilometer.
In the military industry, phosphorus has found a place, just like an igniter. In this role, the element appears and for peaceful purposes. So, red modifications are used in the manufacture of matches. They are lubricated with steam phosphorus sulfur, that is, sulfide of the 15th element.
Phosphorus chloride is needed in the manufacture of plasticizers. So called additives that increase the plasticity of plastics and other polymers. Chloride is also bought by farmers. They mix the substance with insecticides.
They are used to kill pests in the fields, in particular insects. Sprayed planting and pesticides. They already have a duet calcium phosphorusor phosphides.
If insects are killed using phosphorus mixtures, then plants are cultivated. So couples nitrogen phosphorusand potassium phosphorus- regular fertilizers. The 15th element nourishes plantations, accelerates their development, and increases productivity. Phosphorus is necessary for man.
In bones, nucleic chains, proteins, it is hidden approximately 800 grams. No wonder the element was first obtained by distillation of urine. The body's reserves require daily replenishment in the amount of 1.2-1.5 grams. They come with seafood, beans, cheeses and bread.
Phosphorus acidsadded to products and by artificial means. What for? Diluted phosphoric acid serves as a flavor enhancer for syrups, marmalades and carbonated drinks. If E338 is indicated in the composition of the product, we are talking about a compound with the participation of the 15th element of the periodic table.

Phosphorusnature did not associate with his glow. Man, however, emphasized precisely this property. So, the lion's share of the stock of the element goes to the production of paints. Compositions for machines also protect them from corrosion. Inks for glossy surfaces have been invented. There are options for wood, concrete, plastic.
Many synthetic detergents cannot do without the 15th element. They contain magnesium. Phosphorusbinds its ions.
Otherwise, the effectiveness of the compounds is reduced. Without the 15th element, the quality of some steels is also reduced. Their basis is iron. Phosphorus- only.
The additive increases the strength of the alloy. In low alloy steels, phosphorus is needed to facilitate their processing and increase corrosion resistance.
Phosphorus mining
Phosphorus is the 15th in the periodic table, but 11th in terms of prevalence on Earth. The substance is not rare outside the planet. So, meteorites contain from 0.02 to 0.94% of phosphorus. It is also found in soil samples taken from the moon.
Earthly representatives of the element - 200 minerals created by nature on its basis. In its pure form, phosphorus does not occur. Even in the lithosphere, it is represented by orthophosphate, that is, it is oxidized to the highest degree.
To highlight a pure element, industrialists work with calcium phosphate. It is obtained from phosphorites and vorappatites. These are 2 minerals, the richest in the 15th element. After the reduction reaction, 100% phosphorus remains.
The reducing agent is coke, that is, carbon. Calcium, in this case, is bound with sand. All this is done by experts in electric furnaces. That is, the process of phosphorus release refers to electrothermal.
This is the production of white or yellow phosphorus. It all depends on the degree of purification. What needs to be done to convert the product into red, black, metal modifications is described in the chapter “Chemical and physical properties of an element”.
Phosphorus price
There are firms and stores specializing in the supply of chemical raw materials. Phosphorus, as a rule, is offered in packages of 500 grams and kilograms. About 2,000 rubles are being asked for a red modification weighing 1,000 grams.
White phosphorus is offered less often and at a price of about 30-40% cheaper. Black and metal modifications are expensive and are sold, usually to order through large manufacturing enterprises.
Phosphorus and its compounds
Introduction
Chapter I. Phosphorus as an element and as a simple substance
1.1. Phosphorus in nature
1.2. Physical properties
1.3. Chemical properties
1.4. Getting
1.5. Application
Chapter II Phosphorus compounds
2.1. Oxides
2.2. Acids and their salts
2.3. Phosphine
Chapter III. Phosphoric fertilizers
Conclusion
Bibliographic list
Introduction
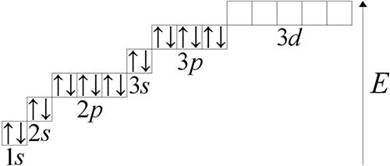
Phosphorus (lat. Phosphorus) P - a chemical element of group V periodic system Mendeleev atomic number 15, atomic mass 30.973762 (4). Consider the structure of the phosphorus atom. At the outer energy level of the phosphorus atom are five electrons. Graphically, it looks like this:1s 2 2s 2 2p 6 3s 2 3p 3 3d 0
In 1699, the Hamburg alchemist X. Brand, in search of a “philosopher's stone”, supposedly able to turn base metals into gold, secreted a white waxy substance that could glow when evaporating urine with coal and sand.
The name "phosphorus" comes from the Greek. “Phos” is light and “phoros” is the carrier. In Russia, the term “phosphorus” was introduced in 1746 by M.V. Lomonosov.
The main phosphorus compounds include oxides, acids and their salts (phosphates, dihydrogen phosphates, hydrophosphates, phosphides, phosphites).
A lot of substances containing phosphorus are found in fertilizers. Such fertilizers are called phosphate.
Chapter I Phosphorus as an element and as a simple substance
1.1 Phosphorus in nature
Phosphorus is one of the common elements. The total content in the earth's crust is about 0.08%. Due to the easy oxidation, phosphorus in nature is found only in the form of compounds. The main phosphorus minerals are phosphorites and apatites, of the latter the most common is fluorapatite 3Ca 3 (PO 4) 2 CaF 2. Phosphorites are widely distributed in the Urals, in the Volga region, Siberia, Kazakhstan, Estonia, Belarus. The largest apatite deposits are located on the Kola Peninsula.
Phosphorus is a necessary element of living organisms. It is present in bones, muscles, in brain tissue and nerves. The molecules of ATP - adenosine triphosphoric acid (ATP - collector and energy carrier) are built from phosphorus. An adult's body contains an average of about 4.5 kg of phosphorus, mainly in combination with calcium.
Phosphorus is also found in plants.
Natural phosphorus consists of only one stable isotope of 31 R. Today, six radioactive isotopes of phosphorus are known.
1.2 Physical properties
Phosphorus has several allotropic modifications - white, red, black, brown, violet phosphorus, etc. The first three of these are the most studied.
White phosphorus - a colorless, yellowish crystalline substance that glows in the dark. Its density is 1.83 g / cm 3. It is not soluble in water, it is well soluble in carbon disulfide. Has a characteristic garlic smell. Melting point 44 ° C, autoignition temperature 40 ° C. To protect white phosphorus from oxidation, it is stored under water in the dark (in the light, it turns into red phosphorus). In the cold, white phosphorus is fragile, at temperatures above 15 ° C it becomes soft and cut with a knife.
Molecules of white phosphorus have a crystal lattice, in the nodes of which are molecules P 4 in the form of a tetrahedron.
Each phosphorus atom is linked by three σ-bonds to the other three atoms.
White phosphorus is poisonous and gives difficult healing burns.
Red phosphorus - powdery substance, dark red, odorless, does not dissolve in water and carbon disulfide, does not glow. Ignition temperature 260 ° C, density 2.3 g / cm 3. Red phosphorus is a mixture of several allotropic modifications that differ in color (from scarlet to purple). The properties of red phosphorus depend on the conditions for its production. Not poisonous.
Black phosphorus by appearance similar to graphite, greasy to the touch, has semiconductor properties. The density of 2.7 g / cm 3.
Red and black phosphors have an atomic crystal lattice.
1.3 Chemical properties
Phosphorus is non-metal. In compounds, it usually exhibits an oxidation state of +5, less commonly, +3 and –3 (only in phosphides).
Reactions with white phosphorus are easier than with red.
I. Interaction with simple substances.
1. Interaction with halogens:
2P + 3Cl 2 \u003d 2PCl 3 (phosphorus (III) chloride),
PCl 3 + Cl 2 \u003d PCl 5 (phosphorus (V) chloride).
2. Interaction with non-metals:
2P + 3S \u003d P 2 S 3 (phosphorus (III) sulfide.
3. Interaction with metals:
2P + 3Ca \u003d Ca 3 P 2 (calcium phosphide).
4. Interaction with oxygen:
4P + 5O 2 \u003d 2P 2 O 5 (phosphorus (V) oxide, phosphoric anhydride).
II. Interaction with complex substances.
1.4 Receipt
Phosphorus is obtained from crushed phosphorites and apatites, the latter are mixed with coal and sand and calcined in furnaces at 1500 ° C:
2Ca 3 (PO 4) 2 + 10C + 6SiO 2
6CaSiO 3 + P 4 + 10CO.Phosphorus is released in the form of vapors that condense in the receiver under water, and white phosphorus forms.
When heated to 250-300 ° C without access to air, white phosphorus turns into red.
Black phosphorus is obtained by prolonged heating of white phosphorus at a very high pressure (200 ° C and 1200 MPa).
1.5 Application
Red phosphorus is used in the manufacture of matches (see. Figure). It is part of the mixture applied to side surface matchbox. The main component of the composition of the match head is the Bertolet salt KClO 3. From the friction of the head of a match on a plastered box, phosphorus particles in the air ignite. As a result of the oxidation of phosphorus, heat is generated, leading to the decomposition of the Berthollet salt.
KCl +.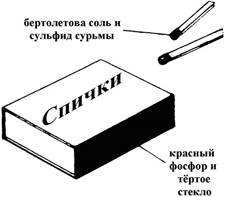
The oxygen produced contributes to the ignition of the match head.
Phosphorus is used in metallurgy. It is used to obtain conductors and is part of some metallic materials, such as tin bronzes.
Phosphorus is also used in the production of phosphoric acid and pesticides (dichlorvos, chlorophos, etc.).
White phosphorus is used to create smoke screens, since white smoke forms during its burning.
Chapter II . Phosphorus compounds
2.1 Oxides
Phosphorus forms several oxides. The most important of them are phosphorus oxide (V) P 4 O 10 and phosphorus oxide (III) P 4 O 6. Often their formulas are written in a simplified form - P 2 O 5 and P 2 O 3. The tetrahedral arrangement of phosphorus atoms is retained in the structure of these oxides.
Phosphorus oxide (III) P 4 O 6 is a waxy crystalline mass that melts at 22.5 ° C and turns into a colorless liquid. Toxic.
When dissolved in cold water forms phosphorous acid:
P 4 O 6 + 6H 2 O \u003d 4H 3 PO 3,
and in the reaction with alkalis, the corresponding salts (phosphites).
Strong reducing agent. When interacting with oxygen, it is oxidized to P 4 O 10.
Phosphorus (III) oxide is obtained by the oxidation of white phosphorus with a lack of oxygen.
Phosphorus oxide (V) P 4 O 10 is a white crystalline powder. Sublimation temperature 36 ° C. It has several modifications, one of which (the so-called volatile) has a composition of P 4 O 10. The crystal lattice of this modification is composed of P 4 O 10 molecules bonded to each other by weak intermolecular forces, which are easily broken when heated. Hence the volatility of this species. Other modifications are polymeric. They are formed by infinite layers of tetrahedra RO 4.
When P 4 O 10 reacts with water, phosphoric acid is formed:
P 4 O 10 + 6H 2 O \u003d 4H 3 PO 4.
Being acid oxide, P 4 O 10 reacts with basic oxides and hydroxides.
Formed during high-temperature oxidation of phosphorus in excess oxygen (dry air).
Due to its exceptional hygroscopicity, phosphorus (V) oxide is used in laboratory and industrial technology as a drying and dehydrating agent. In its draining action, it surpasses all other substances. Chemically bound water is taken from anhydrous perchloric acid to form its anhydride:
4HClO 4 + P 4 O 10 \u003d (HPO 3) 4 + 2Cl 2 O 7.
2.2 Acids and their salts
but) Phosphoric acid H 3 PO 3. Anhydrous phosphorous acid H 3 PO 3 forms crystals with a density of 1.65 g / cm 3, melting at 74 ° C.
Structural formula:
.When heating anhydrous H 3 PO 3, a disproportionation reaction occurs (self-oxidation-self-reduction):
4H 3 PO 3 \u003d PH 3 + 3H 3 PO 4.
Salts of phosphorous acid - phosphites . For example, K 3 PO 3 (potassium phosphite) or Mg 3 (PO 3) 2 (magnesium phosphite).
Phosphorous acid H 3 PO 3 is obtained by dissolving phosphorus (III) oxide in water or by hydrolysis of phosphorus (III) chloride RCl 3:
PCl 3 + 3H 2 O \u003d H 3 PO 3 + 3HCl.
b) Phosphoric acid (orthophosphoric acid) H 3 PO 4.
Anhydrous phosphoric acid is a clear, transparent crystals that, at room temperature, dissolve in air. Melting point 42.35 ° C. Phosphoric acid forms solutions of any concentration with water.
The following structural formula corresponds to phosphoric acid:
.Phosphoric acid reacts with metals located in the range of standard electrode potentials to hydrogen, with basic oxides, with bases, with salts of weak acids.
In the laboratory phosphoric acid obtained by oxidation of phosphorus with 30% nitric acid:
3P + 5HNO 3 + 2H 2 O \u003d 3H 3 PO 4 + 5NO.
In industry, phosphoric acid is obtained in two ways: extraction and thermal. At the heart of extraction method processing of ground natural phosphates with sulfuric acid lies:
Ca 3 (PO 4) 2 + 3H 2 SO 4 \u003d 2H 3 PO 4 + 3CaSO 4 ↓.
Phosphoric acid is then filtered off and concentrated by evaporation.
Thermal method consists in the restoration of natural phosphates to free phosphorus, followed by its combustion to P 4 O 10 and dissolution of the latter in water. The phosphoric acid produced by this method is characterized by a higher purity and increased concentration (up to 80% by weight).
Phosphoric acid is used for the production of fertilizers, for the preparation of reagents, organic substances, to create protective coatings on metals. Purified phosphoric acid is needed for preparation pharmaceutical preparationsfeed concentrates.
Phosphoric acid is not a strong acid. As a tribasic acid, in aqueous solution dissociates in steps. Dissociation along the first stage is easier.
H + + (dihydrogen phosphate ion); H ++ (hydrophosphate ion); H + + (phosphate ion).The total ionic equation for the dissociation of phosphoric acid:
3H ++.Phosphoric acid forms three rows of salts:
a) K 3 PO 4, Ca 3 (PO 4) 2 - trisubstituted, or phosphates;
b) K 2 HPO 4, CaHPO 4 - bisubstituted, or hydrophosphates;
c) KH 2 PO 4, Ca (H 2 PO 4) 2 - monosubstituted, or dihydrogen phosphates.
Monosubstituted phosphates have an acid reaction, bisubstituted - slightly alkaline, trisubstituted - alkaline.
All alkali metal and ammonium phosphates are soluble in water. Of the calcium salts of phosphoric acid, only calcium dihydrogen phosphate dissolves in water. Calcium hydrogen phosphate and calcium phosphate are soluble in organic acids.
When heated, phosphoric acid first loses water, the solvent, then dehydration of phosphoric acid begins and diphosphoric acid is formed:
2H 3 PO 4 \u003d H 4 P 2 O 7 + H 2 O.
A significant part of phosphoric acid is converted to diphosphoric acid at a temperature of about 260 ° C.
at) Phosphoric acid (hypophosphoric acid) H 4 P 2 O 6.
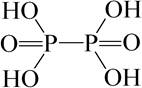 .
.
H 4 P 2 O 6 is a tetrabasic acid of medium strength. During storage, hypophosphoric acid gradually decomposes. When heated, its solutions turn into H 3 PO 4 and H 3 PO 3.
Formed by the slow oxidation of H 3 PO 3 in air or the oxidation of white phosphorus in moist air.
d) Hypophosphorous acid (hypophosphorous acid) H 3 PO 2. This acid is monobasic, strong. Hypophosphorous acid corresponds to the following structural formula:
 .
.
Hypophosphites - salts of hypophosphorous acid - usually soluble in water.
Hypophosphites and H 3 PO 2 are vigorous reducing agents (especially in an acidic environment). Their valuable feature is the ability to restore the dissolved salts of certain metals (Ni, Cu, etc.) to a free metal:
+ 2H 2 O → Ni 0 + + 6H +.Hypophosphorous acid is obtained by decomposition of calcium or barium hypophosphites with sulfuric acid:
Ba (H 2 PO 2) 2 + H 2 SO 4 \u003d 2H 3 PO 2 + BaSO 4 ↓.
Hypophosphites are formed by boiling white phosphorus in suspensions of calcium or barium hydroxides.
2P 4 (white) + 3Ba (OH) 2 + 6H 2 O \u003d 2PH 3 + 3Ba (H 2 PO 2) 2.
2.3 Phosphine
Phosphine PH 3 - a compound of phosphorus with hydrogen - a colorless gas with a sharp unpleasant garlic odor, readily soluble in water (does not chemically interact with it), is very toxic. In air, clean and dry phosphine ignites when heated above 100-140 ° C. If phosphine contains diphosphine impurities P 2 H 4, it spontaneously ignites in air.
When reacted with some strong acids, phosphine forms phosphonium salts, eg:
PH 3 + HCl \u003d PH 4 Cl (phosphonium chloride).
The structure of the phosphonium cation [PH 4] + is similar to the structure of the ammonium cation +.
Water decomposes phosphonium salts to form phosphine and hydrogen halide.
Phosphine can be obtained by the interaction of phosphides with water:
Ca 3 P 2 + 6H 2 O \u003d 3Ca (OH) 2 + 2PH 3.
And the last one. When phosphorus interacts with metals, salts are formed - phosphides . For example, Ca 3 P 2 (calcium phosphide), Mg 3 P 2 (magnesium phosphide).
Chapter III Phosphoric fertilizers
Compounds of phosphorus, as well as nitrogen, constantly undergo transformations in nature - the phosphorus cycle in nature occurs. Plants extract phosphates from the soil and turn them into complex phosphorus-containing organic matter. These substances with plant food enter the animal organism - the formation of protein substances of the nervous and muscle tissues, calcium phosphates in the bones, etc. After the death of animals and plants, phosphorus compounds decompose under the influence of microorganisms. As a result, phosphates are formed. Thus, the cycle expressed by the scheme ends:
P (living organisms)
P (soil).This cycle is disturbed when phosphorus compounds are removed from the crop. The lack of phosphorus in the soil is practically not compensated naturally. Therefore, phosphorus fertilizers must be added.
As you know, mineral fertilizers are simple and complex. To simple include fertilizers containing one nutrient element. Complex fertilizers contain several nutrients.
How do phosphoric fertilizers get in industry? Natural phosphates do not dissolve in water, but are poorly soluble in soil solutions and poorly absorbed by plants. The processing of natural phosphates into water-soluble compounds is the task of the chemical industry. The fertilizer nutrient content of phosphorus is estimated by the content of phosphorus oxide (V) P 2 O 5.
Main component phosphate fertilizer - dihydro- or hydrophosphates of calcium. Phosphorus is part of many organic compounds in plants. Phosphorus nutrition regulates the growth and development of plants. The most common phosphate fertilizers include:
1. Phosphorite flour - fine white powder. Contains 18-26% P 2 O 5.
It is obtained by grinding phosphate Ca 3 (PO 4) 2.
Phosphate rock can be absorbed only on podzolic and peat soils containing organic acids.
2. Simple superphosphate - gray fine-grained powder. Contains up to 20% P 2 O 5.
It is obtained by the interaction of natural phosphate with sulfuric acid:
Ca 3 (PO 4) 2 + 2H 2 SO 4 \u003d Ca (H 2 PO 4) 2 + 2CO 4.
superphosphate
In this case, a mixture of salts of Ca (H 2 PO 4) 2 and CaSO 4 is obtained, which is well absorbed by plants on any soil.
3. Dual superphosphate (color and appearance similar to simple superphosphate).
It is obtained by acting on natural phosphate of phosphoric acid:
Ca 3 (PO 4) 2 + 4H 3 PO 4 \u003d 3Ca (H 2 PO 4) 2.
Compared to simple superphosphate, it does not contain CaSO 4 and is a significantly more concentrated fertilizer (contains up to 50% P 2 O 5).
4. Precipitate - contains 35-40% P 2 About 5.
It is obtained by neutralizing phosphoric acid with a solution of calcium hydroxide:
H 3 PO 4 + Ca (OH) 2 \u003d CaHPO 4 2H 2 O.
It is used on acidic soils.
5. Bone flour . Obtained by processing bones of domestic animals, contains Ca 3 (PO 4) 2.
6. Ammophos - a complex fertilizer containing nitrogen (up to 15% K) and phosphorus (up to 58% P 2 O 5) in the form of NH 4 H 2 PO 4 and (NH 4) 2 NRA 4. It is obtained by neutralizing phosphoric acid with ammonia.
Conclusion
And in conclusion, I would like to say the biological significance of phosphorus. Phosphorus is an integral part tissues of human organisms, animals and plants. In the human body, most of the phosphorus is bound to calcium. To build a skeleton, a child needs as much phosphorus as calcium. In addition to bones, phosphorus is found in the nervous and brain tissues, blood, and milk. In plants, as in animals, phosphorus is part of proteins.
ATP, adenosine triphosphoric acid, which serves as a collector and carrier of energy, as well as nucleic acids, DNA and RNA, transmitting the hereditary properties of the body, is built from phosphorus that enters the human body with food, mainly with eggs, meat, milk and bread. ATP is most intensively consumed in actively working organs of the body: in the liver, muscles, and brain. No wonder the famous mineralogist, one of the founders of the science of geochemistry, academician AE Fersman called phosphorus "an element of life and thought."
As indicated, phosphorus exists in nature in the form of compounds contained in the soil (or dissolved in natural waters) Phosphorus is extracted from the soil by plants, and animals receive phosphorus from plant foods. After the death of plant and animal organisms, phosphorus again passes into the soil. So the phosphorus cycle is carried out in nature.
References:
1. Akhmetov N.S. Chemistry grade 9: textbook. for general education. textbook. institutions. - 2nd ed. - M.: Education, 1999. - 175 p.: Ill.
2. Gabrielyan O.S. Chemistry grade 9: textbook. for general education. textbook. institutions. - 4th ed. - M .: Drofa, 2001 .-- 224 p.: Ill.
3. Gabrielyan O.S. Chemistry grades 8–9: method. allowance. - 4th ed. - M.: Bustard, 2001 .-- 128 p.
4. Eroshin D.P., Shishkin E.A. Methodology for solving problems in chemistry: textbook. allowance. - M .: Education, 1989 .-- 176 p .: ill.
5. Kremenchug M. Chemistry: Handbook of a student. - M .: Filol. Society "WORD": LLC "Publishing house AST", 2001. - 478 p.
6. Kritsman V.A. A book to read on inorganic chemistry. - M .: Education, 1986.- 273 p.
Arsenic, antimony and bismuth sulfides: their relationship to acids and to a solution of ammonium sulfide. Thioacids and their salts.
Arsenic, antimony and bismuth halides: their preparation and hydrolysis. Thio acids and thiosols.
Arsenic, antimony, bismuth.
Arsenic and antimony have a number of allotropic modifications. The most stable metal forms are gray (As) and silver-white (Sb). These are fragile islands. Bismuth is silver-white with a faint pink tint. Less fragile than antimony. In the subgroup, the compounds become more stable from top to bottom. s.o. + 3.In general, for these el-ss of Khar-ni so. from -3 to + 5. Found mainly in the form of sulfides. The preferred method of obtaining:
Sulfide (calcination) → Oxide (о С, t) → Element
Chemical properties:
Reactions simple substances with sulfuric to-that:
4As + 6H2SO4 (conc) \u003d As4O6 + 6SO2 + 6H2O
2Sb + 6H2SO4 (conc) \u003d Sb2 (SO4) 3 + 3SO2 + 6H2O (Bi)
Bi is passive in HNO3 (conc). Mutual interaction with nitrogen:
As + 5HNO3 (conc) \u003d H3AsO4 + 5NO2 + H2O
2Sb + 10HNO3 (conc) \u003d Sb2O5 + 10NO2 + 5H2O
As + HNO3 (dec) + H2O \u003d H3AsO3 + NO
4Sb + 4HNO3 (dec) \u003d Sb4O6 + 4NO + 2H2O
Bi + 4HNO3 (dec) \u003d Bi (NO3) 3 + NO + 2H2O
Metal SbiBi can react with HCl (conc) in the presence of an oxidizing agent
2Sb + 12HCl + 3H2O2 \u003d 2H3 + 6H2O.
In nature, As, Sb and Bi are found in the form of sulfides As2S3, Sb2S3, Bi2S3
Very rarely, these elements are found in a native form. Natural sulfides burn and reversible oxides are reduced by coke:
2As2S3 + 9O2 \u003d 2As2O3 + 6SO2,
2As2O3 + 2C \u003d As4 + 3CO.
At room temperature, As, Sb and Bi only react with halogens, forming trihalides (EG 3), and in the case of antimony and pentahalides (EG 5).). When As, Sb, and even Bi are sintered with active Me powder, arsenides, antimonides, and bismuthides are formed. (Ca3As2, K3Sb)
Sulfides: (E2S3 and E2S5)
Get interaction. pr.vesch-v, or interaction. respectively. conn. in a strongly acidic environment with H2S:
2As + 3S → As2S3
2Na3AsO4 + 6HCl + 5H2S \u003d As2S5 ↓ + 6NaCl + 8H2O
Bi2S3 is sulfide, the remaining compounds are thioanhydrides. They have very small values \u200b\u200bof PR, insoluble in min. acids. These com. can be put into a soluble state either by the action of acid oxidizing agents, for example:
Sb2S3 + 28HNO3 (conc.) \u003d Sb2O5 + 28NO2 + 3H2SO4 + 11H2O
Bi2S3 + 24HNO3 (conc.) \u003d Bi2 (SO4) 3+ 24NO2 + 12H2O
Or in u. solutions:
Sb2S3 + 6KOH \u003d K3SbS3 + K3SbO3 + 3H2O
Sb2S5 + 8KOH \u003d K3SbS4 + K3SbO4 + K2¬S + 4H2O
Sb2S5 + 16KOH \u003d 2K3SbO4 + 5K2S + 8H2O
Sulfides As, Sb, and Bi exhibit some analogy of properties with oxides of the same elements. Just as the oxides of As and Sb in the interaction. with alkalis give salts of acids H3EO3 or H3EO4, their sulfur derivatives arr. with soluble sulfides Me salts of the corresponding thio acids (analogue for antimony sulfide)
3 (NH4) 2S + As2S3 \u003d 2 (NH4) 3AsS3 and
3 (NH4) 2S + As2S5 \u003d 2 (NH4) 3AsS4
Bi2S3 with soluble sulfur salts practically does not react. This sulfide, therefore, behaves similarly to an oxide almost insoluble in alkalis (Bi2O3).
Halides:
Halides As, Sb and Bi are easily formed by direct interaction of elements. EH3 type halides are known for all electrical and Hal halides; EH5 type halides are more or less stable only fluorine derivatives and SbCl5
The halides EG3 and EG5 are strongly hydrolyzed in aqueous solutions; AsCl3 and SbCl5 - almost completely:
AsCl3 + 2H2O \u003d HAsO2 + 3HCl
2SbCl5 + 5H2O \u003d Sb2O5 + 10HCl
Halides Sb (III) and salts of Bi (III) are hydrolyzed to form sparingly soluble oxosalts:
SbCl3 + H2O \u003d SbOCl ↓ (antimonyl chloride) + 2HCl
Bi (NO3) 3 + H2O \u003d BiONO3 ↓ (bismuthyl nitrate) + 2HNO3
BiI3 is soluble in excess iodide, and an unstable complex forms, which is destroyed even by diluting the solution:
BiI3 + KI (g) \u003d K
The Bi3 + ion is capable of being reduced to a metal in solution:
3K2 + 2Bi (NO3) 3 + 6KOH \u003d 3K2 + 2Bi ↓ + 6KNO3
Phosphorus - a chemical element with atomic number 15. Located in the V group of the periodic system D.I. Mendeleev. Chemical formula phosphorus R.
Phosphorus got its name from the Greek phosphoros, which means "luminiferous."
Phosphorus is quite common in the earth's crust. Its content is 0.08-0.09% of the total mass of the earth's crust. And in seawater phosphorus contains 0.07 mg / L.
Phosphorus has a high chemical activity, so it does not occur in a free state. But then it forms almost 190 minerals. Phosphorus is called the element of life. It is contained in green plants, animal tissues, proteins and other important chemical compounds.
Phosphorus modifications
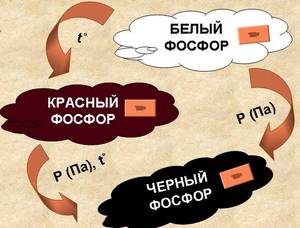
Known that some chemical elements can exist in the form of two or more simple substances that differ in their structure and properties. This phenomenon is called allotropy. So, phosphorus has several allotropic modifications. All these modifications are different in their properties. The most common are white phosphorus, yellow phosphorus, red phosphorus, black phosphorus.
White phosphorus - a simple substance of white color. Him molecular formula P 4. In appearance, white phosphorus is similar to paraffin. It is deformed even with little effort and is easily cut with a knife. In the dark, a pale green glow emanating from phosphorus is noticeable. This phenomenon is called chemiluminescence.
White phosphorus - chemically active substance. It is easily oxidized by oxygen and readily soluble in organic solvents. Therefore, it is stored in special inert media that do not enter into chemical reactions. White phosphorus melts at a temperature of +44.1 ° C. White phosphorus is a very toxic substance.
Yellow phosphorus - This is crude white phosphorus, or white phosphorus with impurities. Melting point +34 ° C, boiling point +280 ° C. Like white, yellow phosphorus does not dissolve in water. It is oxidized and flammable in air. He also has the phenomenon of chemiluminescence.
Red phosphorus obtained by heating white phosphorus to high temperatures. Formula of red phosphorus P n. It is a polymer of complex structure. Depending on the production conditions, the color of red phosphorus can vary from light red to dark brown. Chemically red phosphorus is much less active than white. It dissolves only in molten lead and bismuth. Does not ignite in air. This can happen only when heated to 240-250 about With the sublimation of it in a white form of phosphorus. But it can self-ignite upon impact or friction. The phenomenon of chemiluminescence in red phosphorus is not observed. It does not dissolve in water, benzene, carbon disulfide. It is soluble only in phosphorus tribromide. When stored in air, it gradually oxidizes. Therefore, it is stored in a sealed container.
Red phosphorus is almost non-toxic. Therefore, it is he who is used in the production of matches.
Black phosphorus outwardly similar to graphite. Black phosphorus was first obtained in 1914 from white phosphorus at a pressure of 20 thousand atmospheres (2 · 10 9 Pa) and a temperature of 200 ° C. Black phosphorus melts at a temperature of 1000 ° C and a pressure of 18 · 10 5 Pa. Black phosphorus does not dissolve in input or in organic solvents. It begins to burn only if it is heated to a temperature of +400 ° C in pure oxygen. Black phosphorus has the properties of semiconductor materials.
Chemical properties of elemental phosphorus

1. Elemental phosphorus is oxidized by oxygen
In an environment with excess oxygen
4P + 5O 2 → 2P 2 O 5
With a lack of oxygen
4P + 3O 2 → 2P 2 O 3
2. Interacts with metals, forming phosphides when heated.
3Mg + 2P → Mg 3 P 2
3. Interacts with non-metals
2P + 5Cl 2 → 2PCl 5
4. At a temperature of +500 o C interacts with water vapor
8Р + 12Н 2 О → 5РН 3 + 3Н 3 РО 4
Phosphorus
The main consumer of phosphorus is agriculture. A large amount of all the phosphorus produced is used for the production of phosphate fertilizers: phosphate rock, simple and double superphosphates, complex nitrogen-phosphorus fertilizers. Phosphorus is widely used in the production of synthetic detergentsphosphate glasses for processing and dyeing of natural and synthetic fibers. In medicine, phosphorus preparations are used as medicines.
According to some literary data, the method of obtaining Phosphorus was known to Arab alchemists of the 12th century. But the generally accepted date for the discovery of Phosphorus is 1669, when X. Brand (Germany), upon calcining with sand the dry residue from the evaporation of urine and subsequent distillation without access to air, received a substance glowing in the dark, called first “cold fire”, and later Phosphorus from Greek. phosphoros - luminiferous. Soon, the method of obtaining phosphorus became known to German chemists - I. Kraft, I. Kunkel; in 1682, this method was published. In 1743, A. S. Marggraf developed the following method for the production of Phosphorus: a mixture of lead chloride with urine was evaporated to dryness and heated until the evolution of volatile products ceased; the residue was mixed with charcoal in powder and distilled in a clay retort; Phosphorus vapors condensed in a receiver with water. The simplest method for obtaining phosphorus by calcining bone ash with coal was proposed only in 1771 by C. Scheele. The elemental nature of Phosphorus was established by A. Lavoisier. In the 2nd half of the 19th century, industrial production of phosphorus from phosphorites in retort furnaces arose; at the beginning of the 20th century they were replaced by electric furnaces.
The distribution of phosphorus in nature. The average phosphorus content in the earth's crust (clarke) is 9.3 · 10 -2% by weight; in medium-sized rocks 1.6 · 10 -1, in basic rocks 1.4 · 10 -1, less in granites and other acidic igneous rocks - 7 · 10 -2 and even less in ultrabasic rocks (mantle) - 1.7 · 10 2%; in sedimentary rocks from 1.7 · 10 -2 (sandstones) to 4 · 10 -2% (carbonate rocks). Phosphorus takes part in magmatic processes and migrates vigorously in the biosphere. Both processes are associated with its large accumulations, forming industrial deposits of apatite and phosphorite. Phosphorus is an extremely important biogenic element; it is accumulated by many organisms. Many processes of phosphorus concentration in the earth's crust are associated with biogenic migration. Phosphorus easily precipitates from water in the form of insoluble minerals or is captured by living matter. Therefore, in sea water only 7 · 10 -6% of Phosphorus. About 180 phosphorus minerals are known, mainly various phosphates, of which calcium phosphates are most common.
Physical properties of Phosphorus. Elemental Phosphorus exists in the form of several allotropic modifications, the main of which are white, red and black.
White Phosphorus - a waxy, transparent substance with a characteristic odor, is formed during the condensation of the phosphorus vapor. White Phosphorus in the presence of impurities - traces of red Phosphorus, arsenic, iron, etc. - is colored yellow, therefore, commodity white phosphorus called yellow. There are two forms of white phosphorus: the α and β forms. α-modification is a crystal of a cubic system (a \u003d 18.5 Å); density 1.828 g / cm 3, mp 44.1 ° C, t bale 280.5 ° C, heat of fusion 2.5 kJ / mol P 4 (0.6 kcal / mol P 4), heat of vaporization 58.6 kJ / mol P 4 (14.0 kcal / mol P 4), vapor pressure at 25 ° C 5.7 n / m 2 (0.043 mmHg). The coefficient of linear expansion in the temperature range from 0 to 44 ° C is 12.4 · 10 -4, thermal conductivity of 0.56 W / (m · K) at 25 ° C. By its electrical properties, α-white Phosphorus is close to dielectrics: the band gap is about 2.1 eV, the electrical resistivity is 1.54 · 10 11 ohm · cm, it is diamagnetic, and the specific magnetic susceptibility is -0.86 · 10 -6. Brinell hardness 6 Mn / m 2 (0.6 kgf / mm 2). The α-form of white phosphorus dissolves well in carbon disulfide, worse in liquid ammonia, benzene, carbon tetrachloride, etc. At -76.9 ° C and a pressure of 0.1 Mn / m 2 (1 kgf / cm 2), the α-form passes into the low-temperature β-form (density 1.88 g / cm 3). With increasing pressure to 1200 Mn / m 2 (12000 kgf / cm 2), the transition occurs at 64.5 ° C. β-Form - crystals with birefringence. White Phosphorus is poisonous: it spontaneously ignites in air at a temperature of about 40 ° C, so it should be stored under water (solubility in water at 25 ° C 3.3 · 10 -4%). By heating white Phosphorus without air at 250-300 ° C for several hours, red Phosphorus is obtained. The transition is exothermic, accelerated by ultraviolet rays, as well as impurities (iodine, sodium, selenium). Ordinary commodity red Phosphorus is almost completely amorphous; It has a dark brown to violet color. With prolonged heating, it can irreversibly transform into one of the crystalline forms (triclinic, cubic, and others) with various properties: density from 2.0 to 2.4 g / cm 3, mp from 585 to 610 ° C at a pressure of several tens of atmospheres , sublimation temperature from 416 to 423 ° С, electrical resistivity from 10 9 to 10 14 ohm · cm. Red Phosphorus in air does not ignite spontaneously, up to a temperature of 240-250 ° C, but spontaneously ignites upon friction or impact; insoluble in water, as well as in benzene, carbon disulfide and others, soluble in phosphorus tribromide. At the sublimation temperature, red Phosphorus turns into steam, upon cooling of which mainly white Phosphorus is formed.
When white Phosphorus is heated to 200-220 ° C under a pressure of (1.2-1.7) · 10 3 Mn / m 2 [(12-17) · 10 3 kgf / cm 2] black Phosphorus is formed. This transformation can be carried out without pressure, but in the presence of mercury and a small amount of black phosphorus crystals (seeds) at 370 ° C for 8 days. Black Phosphorus represents crystals of a rhombic structure (a \u003d 3.31 Å, b \u003d 4.38 Å, c \u003d 10.50 Å), the lattice is constructed of fibrous layers with a pyramidal arrangement of atoms characteristic of Phosphorus, density 2.69 g / cm 3, t PL about 1000 ° C under a pressure of 1.8 · 10 3 Mn / m 2 (18 · 10 3 kgf / cm 2). In appearance, black phosphorus is similar to graphite; semiconductor: band gap 0.33 eV at 25 ° C; has a specific electrical resistance of 1.5 ohm · cm, a temperature coefficient of electrical resistance of 0.0077, diamagnetic, specific magnetic susceptibility of -0.27 · 10 -6. When heated to 560-580 ° C, under the pressure of its own vapor, it turns into red Phosphorus. Black Phosphorus is inactive, hardly ignites when set on fire, so it can be safely machined in air.
The atomic radius of Phosphorus is 1.34 Å, ionic radii: P 5+ 0.35 Å, P 3+ 0.44 Å, P 3 - 1.86 Å.
Phosphorus atoms are combined into diatomic (P 2), tetratomic (P 4) and polymer molecules. The most stable under normal conditions are polymer molecules containing long chains of interconnected P 4 tetrahedrons. In liquid, solid form (white Phosphorus) and in vapors below 800 ° С Phosphorus consists of P 4 molecules. At temperatures above 800 ° C, P 4 molecules dissociate into P 2, which, in turn, decompose into atoms at temperatures above 2000 ° C. Only white phosphorus consists of P 4 molecules; all other modifications are polymers.
Chemical properties of phosphorus. The configuration of the outer electrons of the phosphorus atom 3s 2 3p 3; oxidation states +5, +3, and -3 are most characteristic in compounds. Like nitrogen, phosphorus in the compounds is mainly covalent. There are very few ionic compounds like Na 3 P, Ca 3 P 2 phosphides. Unlike nitrogen, Phosphorus has free 3d orbitals with rather low energies, which leads to the possibility of increasing the coordination number and the formation of donor – acceptor bonds.
Phosphorus is chemically active, white Phosphorus has the greatest activity; red and black phosphorus in chemical reactions much more passive. The oxidation of white phosphorus occurs by a mechanism chain reactions. Oxidation of Phosphorus is usually accompanied by chemiluminescence. When Phosphorus is burned in an excess of oxygen, oxide (V) P 4 O 10 (or P 2 O 5) is formed, with a deficiency, mainly oxide (III) P 4 O 6 (or P 2 O 3). Spectroscopically proved the existence in pairs of P 4 O 7, P 4 O 8, P 2 O 6, PO and other phosphorus oxides. Phosphorus (V) oxide is produced commercially by burning elemental Phosphorus in excess of dry air. Subsequent hydration of P 4 O 10 leads to the production of ortho- (H 3 PO 4) and poly- (H n + 2 P n O 3n + 1) phosphoric acids. In addition, Phosphorus forms phosphorous acid H 3 PO 3, phosphoric acid H 4 P 2 O 6 and hypophosphorous acid H 3 PO 2, as well as peroxyacids: superphosphoric H 4 P 2 O 8 and mononadphosphoric H 3 PO 5. Salts of phosphoric acids (phosphates) are widely used, to a lesser extent phosphites and hypophosphites.
Phosphorus is directly connected to all halogens with the release of a large amount of heat and the formation of trihalides (PX 3, where X is halogen), pentahalides (PX 5) and oxyhalides (for example, POX 3). When phosphorus and sulfur are fused below 100 ° С, solid solutions are formed on the basis of phosphorus and sulfur, and above 100 ° С an exothermic reaction of formation of crystalline sulfides P 4 S 3, P 4 S 5, P 4 S 7, P 4 S 10, from of which only P 4 S 5 decomposes into P 4 S 3 and P 4 S 7 when heated above 200 ° C, and the rest melt without decomposition. Phosphorus oxysulfides are known: P 2 O 3 S 2, P 2 O 2 S 3, P 4 O 4 S 3, P 6 O 10 S 5 and P 4 O 4 S 3. Compared to nitrogen, phosphorus is less capable of forming compounds with hydrogen. Hydrogen phosphorous phosphine PH 3 and diphosphine P 2 H 4 can only be obtained indirectly. Of the compounds of Phosphorus with nitrogen, nitrides PN, P 2 N 3, P 3 N 5 are known — solid, chemically stable substances obtained by passing nitrogen with phosphorus vapors through an electric arc; polymer phosphonitrile halides - (PNX 2) n (e.g. polyphosphonitrile chloride) obtained by reacting pentahalides with ammonia at different conditions; amidoimidophosphates - compounds, usually polymeric, containing, along with R-O-R bonds P-NH-P bond.
At temperatures above 2000 ° C, Phosphorus reacts with carbon to form PC 3 carbide, a substance that does not dissolve in ordinary solvents and does not interact with acids or alkalis. When heated with metals, phosphorus forms phosphides.
Phosphorus forms numerous organophosphorus compounds.
Getting Phosphorus. Elemental Phosphorus is produced by electrothermal reduction from natural phosphates (apatites or phosphorites) at 1400-1600 ° C with coke in the presence of silica (silica sand):
2Са 3 (РО 4) 2 + 10С + nSiO 2 \u003d P 4 + 10СО + 6СаО · nSiO 2
Pre-crushed and enriched phosphorus-containing ore is mixed in predetermined proportions with silica and coke and loaded into an electric furnace. Silica is necessary to reduce the reaction temperature, as well as increase its speed due to the binding of calcium oxide released during the reduction process to calcium silicate, which is continuously removed in the form of molten slag. Silicates and oxides of aluminum, magnesium, iron and other impurities also pass into slag, as well. ferrophosphorus (Fe 2 P, FeP, Fe 3 P), formed during the interaction of part of the reduced iron with Phosphorus. Ferrophosphorus, as well as small amounts of phosphides of manganese and other metals dissolved in it, are removed from the electric furnace as they accumulate for subsequent use in the production of special steels.
Phosphorus vapors leave the furnace together with gaseous by-products and volatile impurities (CO, SiF 4, PH 3, water vapors, pyrolysis products of organic impurities of the charge and others) at a temperature of 250-350 ° C. After dust removal, the phosphorus-containing gases are sent to condensing units where liquid technical white phosphorus is collected under water at a temperature of at least 50 ° C.
The use of phosphorus. The bulk of the phosphorus produced is processed into phosphoric acid and the phosphorus fertilizers and technical salts (phosphates) obtained on its basis.
White Phosphorus is used in incendiary and smoke shells, bombs; Red Phosphorus - in match production. Phosphorus is used in the production of non-ferrous metal alloys as a deoxidizer. Introduction up to 1% Phosphorus increases the heat resistance of alloys such as fechral, \u200b\u200bchromal. Phosphorus is part of some bronzes, as it increases their fluidity and resistance to abrasion. Phosphides of metals, as well as some non-metals (B, Si, As, etc.) are used in the preparation and alloying of semiconductor materials. Partially, Phosphorus is used to produce chlorides and sulfides, which serve as starting materials for the production of phosphorus-containing plasticizers (for example, tricresyl phosphate, tributyl phosphate and others), medicines, organophosphorus pesticides, and are also used as additives in lubricants and fuel.
Safety precautions. White Phosphorus and its compounds are highly toxic. Work with Phosphorus requires careful sealing of equipment; White phosphorus should be stored under water or in a hermetically sealed metal container. When working with Phosphorus, safety precautions should be strictly observed.
Phosphorus in the body. Phosphorus is one of the most important biogenic elements necessary for the life of all organisms. It is present in living cells in the form of ortho- and pyrophosphoric acids and their derivatives, and is also part of nucleotides, nucleic acids, phosphoproteins, phospholipids, phosphoric esters of carbohydrates, many coenzymes and other organic compounds. Thanks to the features chemical buildings Phosphorus atoms, like sulfur atoms, are capable of forming energy-rich bonds in macroergic compounds: adenosine triphosphoric acid (ATP), creatine phosphate, and others. In the process of biological evolution, it was phosphorus compounds that became the main, universal custodians of genetic information and energy carriers in all living systems. Another important role of phosphorus compounds in the body is that the enzymatic addition of a phosphoryl residue to various organic compounds (phosphorylation) serves as a “pass” for their participation in the metabolism, and, conversely, the removal of the phosphoryl residue (dephosphorylation) excludes these compounds from active metabolism. Phosphorus metabolism enzymes - kinases, phosphorylases and phosphatases. The main role in the conversion of phosphorus compounds in animals and humans is played by the liver. Phosphorus metabolism is regulated by hormones and vitamin D.
Phosphorus content (in mg per 100 g of dry matter) in plant tissues - 230-350, marine animals - 400-1800, terrestrial - 1700-4400, in bacteria - about 3000; in the human body, there is especially a lot of Phosphorus in bone tissue (slightly more than 5000), in brain tissues (about 4000) and in muscles (220-270). The daily human need for Phosphorus is 1-1.2 g (in children it is higher than in adults). Of the foodstuffs, cheese, meat, eggs, and legume crops (peas, beans, and others) are richest in Phosphorus. The balance of phosphorus in the body depends on the general state of metabolism. Violation of phosphorus metabolism leads to profound biochemical changes, primarily in energy metabolism. With a lack of phosphorus in the body, animals and humans develop osteoporosis and other bone diseases, and in plants - phosphorus starvation. The source of phosphorus in wildlife is its inorganic compounds contained in the soil and dissolved in water. Phosphorus is extracted from the soil by plants in the form of soluble phosphates. Animals usually receive a sufficient amount of Phosphorus with food. After the death of organisms, Phosphorus again enters the soil and bottom sediments, thus participating in the circulation of substances. The important role of phosphorus in the regulation of metabolic processes determines the high sensitivity of many enzyme systems of living cells to the action of organophosphorus compounds. This fact is used in medicine in the development of medicinal products, in agriculture in the production of phosphate fertilizers, as well as in the creation of effective insecticides. Many phosphorus compounds are extremely toxic and some of the organophosphorus compounds can be classified as chemical warfare agents (sarin, soman). The radioactive isotope Phosphorus 32 P is widely used in biology and medicine as an indicator in the study of all types of metabolism and energy in living organisms.
Poisoning with Phosphorus and its compounds is observed during their thermoelectric sublimation, work with white Phosphorus, production and use of phosphorus compounds. Organophosphorus compounds that have an anticholinesterase effect are highly toxic. Phosphorus enters the body through the respiratory system, the gastrointestinal tract, and the skin. Acute poisoning is manifested by burning in the mouth and stomach, headache, weakness, nausea, and vomiting. After 2-3 days, pain occurs in the epigastric region, right hypochondrium, jaundice. Chronic poisoning is characterized by inflammation of the mucous membranes of the upper respiratory tract, signs of toxic hepatitis, a violation of calcium metabolism (the development of osteoporosis, brittleness, sometimes necrosis of bone tissue, often on the lower jaw), damage to the cardiovascular and nervous systems. First aid for acute poisoning through the mouth (most common) - gastric lavage, laxative, cleansing enemas, intravenous solutions of glucose, calcium chloride, etc. For skin burns - treat the affected areas with solutions copper sulfate or soda. The eyes are washed with a 2% solution of baking soda. Prevention: compliance with safety regulations, personal hygiene, oral care, preventive examinations of people working with Phosphorus.