Well water requirements. Key indicators of drinking water quality
Most people assume that water coming from wells, is a priori pure and cannot contain harmful impurities. Of course, in most cases this is true, but the reverse also happens. Often, the drinking liquid is contaminated, and it contains a large number of toxins, impurities, bacteria. In this regard, a completely logical question arises: where and how to analyze water from a well and how much does it cost? If you are in doubt about the quality of your drinking water, do not hesitate to conduct a special analysis.

When is verification necessary?
This is a voluntary measure, but there are times in life when its implementation is extremely necessary.
- Acquisition / sale of real estate.
In the first case, you can be sure that you will drink clean water. In the second, increase the value and value of the real estate offer in the eyes of a potential buyer.

- Having health problems.
If you or your loved ones have health problems, use clean water- the guarantee of recovery. After all, many ailments come because of dirty water - indigestion, colds, allergic reactions.

- Conducting preparations for the opening of institutions. If you are planning to open a kindergarten or a health center, a clinic, a sanatorium, checking the liquid for various kinds of impurities is strictly required.
- Carrying out system data calculations. In the event that a decision has been made purchasing an appropriate filtration unit, will require clarification of the degree of water pollution.
- Conclusion of an agreement with the selected laboratory.
- The arrival of a specialist at home in order to select water. This is done in special containers. The place and time of the fence is affixed to them. Two containers are usually used - one for chemical research, the other for microbiological analysis.
- Delivery of the taken samples to laboratory conditions.


- Research directly. First of all, organoleptic factors are checked. After that, specialized chemical analysts are included in the work, who determine the presence of certain substances in the water. chemical substances. Next, they are assisted by microbiologists who determine the number of bacteria.

- At the end of the period of work, conclusions are made, which are issued in documentary form to the customers of the inspection. They receive a protocol with the results of all tests, as well as basic recommendations for eliminating problems.
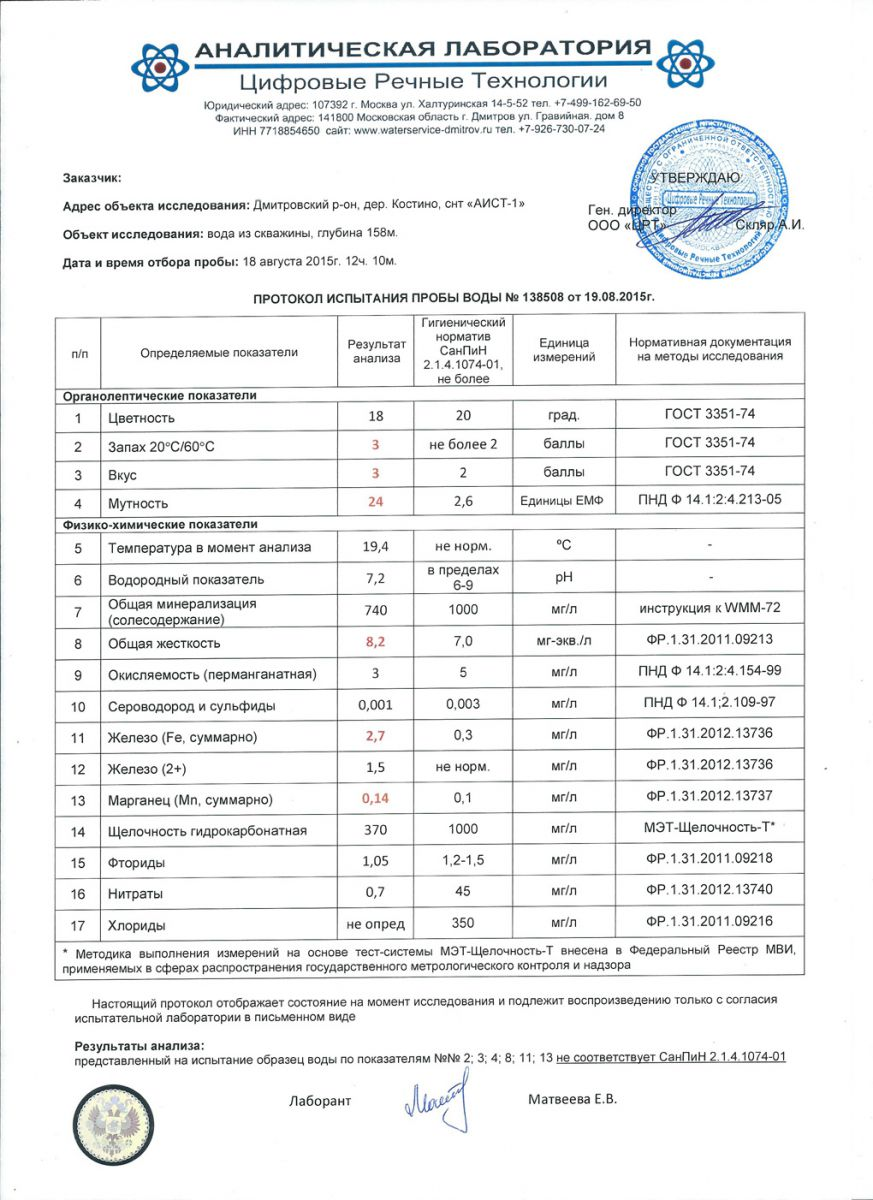
An example of a literate chemical analysis water from an accredited SES laboratory
Important! The timing of the implementation of measures is most often determined by the size of the chosen institution. Small companies conduct an examination in a couple of weeks, while large laboratories cope with the task in 3-7 days of work.
How much does a well water test cost?
In fact, research is not as expensive as it might seem at first glance. In return, the customer receives a huge benefit. The price range for services may vary. But let's look at the average.
On a note! In total, analysis together with sampling costs from 3,000 to 5,000 rubles. If we are talking about an extended examination, it comes at a price of up to 6,000 rubles. As for the full examination, you will have to pay about 9,000 rubles for it. The total amount depends on the list of parameters that are checked, as well as on the list of additional services.
So, we considered the features of the analysis and made special conclusions. It is important to correctly select a laboratory, based on the experience of its work, the professionalism of employees, the quality of the equipment used, and reviews. Only experts will be able to conduct the event as competently and efficiently as possible.

Quality checking aquatic environment before installing cleaning systems
It is worth carrying out these activities on a regular basis, repeating them every few years, because under the influence of natural factors, the composition of water can change.
Important! It is worth doing an analysis of drinking fluid a month after drilling. After all, the impurities that were formed as a result of mounting are leveled at this time.

Where can the analysis be done?
There are plenty of laboratories engaged in the field of such analyzes. Their differences lie in the cost of services, as well as in the direct quality of work. It is best to give preference to large companies that have worked on the market for a long time. Before making a final decision, you should familiarize yourself with the list of tests that are carried out.
Advice! It is also worth clarifying whether the company has its own laboratory and state accreditation. Any institution undertakes to conclude a contract, within which any analyzes and tests will be indicated.
Types of indicators to evaluate
To analyze the general state of the liquid, it is necessary to carry out various tests. They include an organoleptic, microbiological, chemical measure, as well as a comprehensive assessment, which is designed to give the most complete picture of the overall water quality. Traditionally, the lion's share of laboratories carries out verification activities for several indicators.

Ionomer I-510 for determining the activity of hydrogen ions in aqueous solutions
| Index | What does it mean? |
|---|---|
| Hydrogen ion activity | The normal value is in the range of 6-9 units. If the level is exceeded, the water becomes unpleasant and seems to acquire soapy properties. This detracts from its flavor. |
| Rigidity | This is a critical indicator, as it displays the content of calcium and magnesium ions in the drinking liquid. If the water is excessively hard, it harms not only appliances and utensils, but also human health. |
| Dry residue | This parameter reflects the content in water of various dissolved substances of an organic or inorganic nature. |
| The presence of nitrates | The concentration of these harmful elements should not be greater than 45 mg/l. If the parameter is larger, this may indicate that the soil is contaminated with nitrates. |
| sulfates and chlorides | According to regulatory standards, the concentration of the first elements should be no more than 500 mg / l, the second - 350. |
| Oxidability | This indicator means the presence of an acidic environment in the water. It should not exceed 5-7 mg/l. |
| Microbiological research | It involves counting how many microorganisms are contained in 1 ml of liquid. GOST suggests that the water from the well should not contain them at all. |
| Organoleptic research | These indicators make it possible to evaluate such parameters as taste characteristics, transparency factor, color, aroma. |

Event stages
According to the normative indicators of the standards, there are several stages of water analysis.
According to sanitary standards, drinking water must be safe in epidemiological and radiation terms, harmless in chemical composition, and have pleasant organoleptic properties. Therefore, it is advisable to meet the requirements sanitary norms and rules. To select a water purification system from a well or a well, it is important to check the water.
The requirements (standards) that water must comply with are set out in the sanitary norms and rules of the Russian Federation (SanPiN) and international standards of the World Health Organization (WHO), the main provisions of which are given in the table below. And so, consider main indicators of water quality.
Organoleptic properties of water
The organoleptic properties of water include the following characteristics: smell, taste, color and turbidity.
Smell
The smell and taste of water are explained by the presence of natural or artificial pollution in it. The nature of odors and tastes is very different, and can be due to both the presence of certain dissolved salts in water and the content of various chemical and organic compounds.
In addition, it should be noted that the smell and taste can appear in water at several stages: from the original natural water, in the process of water treatment (including in a water heater), during transportation through pipelines. The correct identification of the source of odors and tastes is the key to the success of their elimination.
The magnitude (intensity) of the smell is determined on a 6-point scale. For example, the smell of rotten eggs is due to the presence of hydrogen sulfide (H 2 S) in the water, as well as the presence of sulfate-reducing bacteria that produce this gas, and the putrefactive odor is due to the presence of natural organic compounds in the water. Chemical odors (for example, gasoline, phenol) indicate the anthropogenic nature of pollution.
Taste and taste of water
The taste of water is due to the substances dissolved in water. natural substances, each of which gives the water a certain flavor:
- brackish - sodium chloride;
- bitter - magnesium sulfate;
- sour - dissolved carbon dioxide or dissolved acids.
The pleasant or unpleasant taste of water is provided both by the presence and the concentration of impurities in it.
Chroma
Color refers to the natural color of natural and drinking water. Color indirectly characterizes the presence of some organic and inorganic dissolved substances in water and is one of the important indicators that allow you to choose the right water treatment system.
The color of water is determined by comparison with solutions of a specially prepared color scale (based on certain concentrations of a chromium-cobalt solution) and is expressed in degrees of color of this scale. According to drinking water requirements this indicator should not exceed 20 degrees.
The main "culprits" of water color are organic substances washed out of the soil (mainly humic and fulvic acids). The increased color of the water may also indicate its possible technogenic pollution. The presence of humic acids can lead to a certain biological activity of water, increases the permeability of metal ions in the intestine: iron, manganese, etc.
Turbidity
An indicator characterizing the presence of suspended solids of inorganic origin in water (for example, carbonates various metals, iron hydroxides), organic origin (colloidal iron, etc.), mineral origin (sand, clay, silt), as well as microbiological origin (bacterio-, phyto- or zooplankton). Turbidity is expressed in mg/dm3.
Turbidity can also be due to the presence of various microorganisms on the surface and inside suspended particles, which protect them from both chemical and ultraviolet disinfection water. Therefore, the reduction of turbidity in the process of water purification also contributes to a significant reduction in the level of microbiological contamination.
Chemical indicators of water quality
Chemical indicators characterize chemical composition water. These indicators include the pH value of water, pH, hardness and alkalinity, mineralization (dry residue), anionic and cationic composition (inorganic substances), content organic matter.
Oxidability.
An indicator that characterizes the integral pollution of water, i.e. the content in water of oxidizing organic and inorganic impurities, which under certain conditions are capable of being oxidized by a strong chemical oxidizing agent. The pollutants mentioned above are mainly organic substances - for water from surface sources, and inorganic ions (Fe 2+ , Mn 2+ , etc.) - for water from artesian wells.
There are several types of water oxidizability: permanganate (PMO), bichromate, iodate. As can be seen from the names, at the same time, appropriate oxidizing agents are used to conduct a chemical analysis of water. The oxidizability index - mgO / l - is the amount of milligrams of oxygen equivalent to the amount of the reagent (oxidizing agent) used to oxidize the substances contained in 1 liter of water.
The value of bichromate oxidizability is usually used to determine such an important indicator of water as COD - chemical oxygen demand. COD is used to characterize polluted natural surface waters as well as wastewater. This indicator indicates the degree of biogenic contamination of water.
Bichromate oxidizability makes it possible to obtain a value that most fully characterizes the presence of organic pollutants, with the exception of such chemically inert substances as gasoline, kerosene, benzene, toluene, etc. It is believed that when determining this indicator, up to 90% of organic impurities are oxidized.
In practice, to characterize drinking water, the permanganate oxidizability (PMO) or permanganate index (PMI) is usually used. The higher the PMO value, the higher the concentration of pollutants. Note that the value of permanganate oxidizability is lower than the value obtained for bichromate by about 3 times.
Hydrogen index, pH
pH or pH is the logarithm of the concentration of hydrogen ions, taken with the opposite sign, i.e. pH \u003d -logH + 1. The pH value is determined by the quantitative ratio of H + and OH - ions in water, which are formed during the dissociation of water. If OH - ions predominate in water, which corresponds to a pH value> 7, then the water will have alkaline reaction, and with an increased content of H + ions, which corresponds to pH<7, вода имеет кислую реакцию. В очищенной дистиллированной воде эти ионы уравновешивают друг друга и ее рН приблизительно равен 7.
When any substances are dissolved in water, the balance of the mentioned ions is disturbed, and therefore a change in pH will occur. For example, even when stored in an open container, purified water will have an acidic reaction due to the absorption of carbon dioxide from the air:
CO 2 (gas) + H 2 O ---> + + HCO 3 -
The flow rate can change depending on the pH value. chemical reactions, the degree of corrosive aggressiveness of water, the toxicity of pollutants and many other characteristics.
Typically, the pH level for water used for household and drinking purposes is normalized within the range of 6..9.
dry residue.
This value characterizes the amount of dissolved inorganic and organic substances. First of all, this affects the organoleptic properties of water. It has been established that up to 1000 mg/l water can be used for water consumption.
The amount of dry residue affects the taste of drinking water. A person can use water with a dry residue of up to 1000 mg / l without risk to his health. At greater meaning the taste of water most often becomes unpleasant bitter-salty. It should also be noted that the water low level dry residue, the taste may be absent and it is also not very pleasant to use it.
Rigidity
This indicator characterizes the property of water associated with the content of dissolved salts of alkaline earth metals in it, mainly calcium and magnesium (the so-called "hardness salts").
Water with a high content of such salts is called hard, with a low content - soft.
The numerical expression of water hardness is the concentration of calcium and magnesium cations in it. According to GOST R 52029-2003, hardness is expressed in degrees of hardness (° W), which corresponds to the concentration of the alkaline earth element, numerically equal to 1/2 of its mole, expressed in mg / dm³ (g / m³) (1 ° W \u003d 1 mg-eq / l).
Distinguish temporary (carbonate) hardness due to calcium and magnesium bicarbonates (Ca 2+ and Mg 2+ cations and HCO 3 - anions).
When water is boiled, hydrocarbonate anions react with these cations and form sparingly soluble carbonate salts with them, which precipitate on heating elements in the form of scale white color, called lime in the common people.
Ca 2+ + 2HCO 3 - = CaCO 3 ↓ + H 2 O + CO 2
Temporary hardness can be eliminated by boiling - hence its name.
Permanent (non-carbonate) water hardness caused by the presence of salts that do not precipitate when boiled. Basically, these are sulfates and chlorides of calcium and magnesium (CaSO 4, CaCl 2, MgSO 4, MgCl 2). It should be noted that it is the presence of the CaSO 4 salt, whose solubility decreases with increasing water temperature, that leads to the formation of dense scale.
Water with high hardness causes great harm to household electric heaters, forming scale and thereby causing them to overheat and destroy, forms unpleasant dull deposits on plumbing; soap and shampoos do not foam well in it, and therefore their consumption increases.
Hard water dries out the skin and damages the hair; negatively affects the quality of cooked food, useful material which can form compounds with hardness salts that are poorly absorbed by the body.
Hard water is also harmful to the human body: the risk of developing urolithiasis increases, and water-salt metabolism is disturbed.
Sometimes as a characteristic there is an indicator "full rigidity" water equal to the sum of constant and variable (carbonate) hardness.
Iron
Its toxic effect on the human body is insignificant, but the use of drinking water with a high content of iron can lead to the deposition of its compounds in human organs and tissues.
In the general case, iron can occur in water in free form in the form of divalent and trivalent ions:
Fe2+- usually in artesian wells in the absence of dissolved oxygen. Water with a high content of such iron may initially be transparent (Fe 2+), but upon settling or heating, it acquires a yellowish-brown color. This occurs as a result of the oxidation of dissolved iron to Fe 3+ with the formation of insoluble ferric salts:
Fe3+- is found in surface water sources in the so-called oxidized state, and, as a rule, in an insoluble form.
organic iron
There is another form of the presence of iron in natural water is organic iron. It is found in water different forms and as part of various complex compounds of trivalent iron ions with dissolved inorganic and organic compounds, and, mainly, with salts of humic acids - humates. An increased content of such iron is observed in swamp waters and the water has a brown or brownish color.
Organic iron compounds are usually soluble or have a colloidal structure ( colloidal iron) and are very difficult to remove. Colloidal particles, due to their small size and high surface charge, which do not allow particles to approach each other and prevent their enlargement, preventing the formation of conglomerates, create suspensions in water and do not settle, being in a suspended state, and thereby cause the turbidity of the source water.
The taste of such water has a characteristic unpleasant metallic taste, forms rusty smudges. The presence of colloidal iron in water contributes to the development of ferruterous bacteria, which further worsens the taste of water and causes sedimentation on the inner surface of pipelines and sanitary equipment up to their complete clogging.
Manganese
Manganese is part of many enzymes, hormones and vitamins that affect growth processes, blood formation, and the formation of immunity. However, its increased content in water can have a toxic and mutagenic effect on the human body.
Water with a high manganese content has a metallic taste. Its presence leads to significantly faster wear household appliances and heating systems, since it can accumulate in the form of a black coating on the inner surfaces of pipes, followed by flaking and the formation of a black sediment suspended in water. In addition, an increased content of manganese leads to the formation of black spots on dishes, white linen during washing, stains nails and teeth in a grayish-black color.
There are also "manganese" bacteria, which, like "glandular" bacteria, can grow in such water and become the cause of overgrowth and blockage of pipelines.
Ammonium nitrogen (NH 3 and NH 4 +)
An indicator that most often characterizes the presence of organic substances of animal or industrial origin in water. Sources of ammonium nitrogen are: livestock farms, household wastewater, sewage from agricultural lands, food and chemical industries.
These compounds are mainly degradation products of urea and proteins. The limiting value of the indicator "ammonium nitrogen" is toxicological. According to SanPiN standards, the content of ammonium in water should not exceed 2.0 mg/l.
Microbiological indicators of water quality
The microbiological indicators of the safety of drinking water include the total microbial count, the content of bacteria of the Escherichia coli group (common coliform bacteria and coliphages), spores of sulfite-reducing clostridia and Giardia cysts.
Checking the quality of drinking water is carried out on the basis of the norms of indicators according to the requirements normative documents states.
The table shows the standards of the main quality indicators according to SanPiN sanitary standards Russian Federation indicated in column 3 - SanPiN 2.1.4.1074-01"Hygienic requirements for water quality of centralized drinking water supply systems" and column 4 - SanPiN 2.1.4.1175-02“Hygienic requirements for water quality of non-centralized water supply. Sanitary protection of sources.
It is by these indicators that you should check the quality of water from your source and assess the need for installation. additional equipment for water purification.
For comparison, the standards of the World Health Organization (WHO) are given.
Standards for the main indicators of water quality according to the requirements of the sanitary standards of the Russian Federation and WHO
| Index | Unit rev. | SanPiN 2.1.4. 1074-01 | SanPiN 2.1.4. 1175-02 | WHO |
|---|---|---|---|---|
|
Organoleptic indicators |
||||
|
no more than 2..3 |
||||
|
no more than 2..3 |
||||
|
Chroma |
no more than 30 |
|||
|
Turbidity |
||||
|
or mg/l for kaolin |
||||
|
Chemical indicators |
||||
|
Hydrogen indicator |
||||
|
Dry residue |
||||
|
General hardness |
||||
|
Oil products |
||||
|
Phenolic index |
||||
|
Alkalinity |
mg HCO 3 - /l |
|||
|
inorganic substances |
||||
|
Aluminum (Al3+) |
||||
|
Ammonia nitrogen |
||||
|
Iron (Fe, total) |
||||
|
Manganese (Mn, total) |
||||
|
Nitrates (according to NO 3 -) |
||||
|
Nitrites (according to NO 2 -) |
||||
|
Sulphates (SO 4 2-) |
||||
|
Fluorides (F) |
||||
|
Chlorides (Cl-) |
||||
|
Zinc (Zn2+) |
||||
|
microbiological indicators |
||||
|
Thermotoler. coli bacteria |
Number of bacteria in 100 ml |
|||
|
Common coli bacteria |
Number of bacteria in 100 ml |
|||
|
coliphages |
Number of units in 100 ml |
|||
|
Total microbial count |
The number of microbes in 1 ml |
|||
* - as directed by the Chief State Sanitary Doctor
The list of mandatory water quality indicators for the selection of filters and water purification systems
For right choice water treatment systems, it is necessary to analyze the source water in an accredited laboratory. If it is convenient geographically, bring a water sample. The cost of analysis for 15 physical and chemical indicators is 2,500 rubles, the analysis time is 5-7 working days (slightly longer in summer).
In most cases, an abbreviated chemical analysis of water according to 15 main indicators is sufficient to select filters:
- smack
- smell
- turbidity
- chromaticity
- pH value (pH)
- permanganate oxidizability (PMO)
- total hardness
- iron total
- sulfates
- chlorides
- manganese
- ammonia and NH 4 +
- nitrites
- nitrates
- fluorides
In cases where a shallow well (up to 10-15 meters) or a well fed by surface waters, you should additionally check such indicators as:
- oil products
- surfactants (surfactants)
- phenolic index
And if there is a specific smell in the water, you should additionally check the water for the presence of impurities such as:
- hydrogen sulfide
- total nitrogen
We do not perform microbiological analysis of water. To do this, you need to contact the nearest SES.
Water sampling for analysis
For analysis according to organoleptic and chemical indicators a 1.5 liter water sample is sufficient. Suitable as a container plastic bottle from drinking or mineral water(but in no case from lemonades, beer, etc., which are quite difficult to completely wash off). When taking a sample, turn on the water supply, let the water run for 5..10 minutes, thoroughly rinse the container and cork 2..3 times, draw water under the very neck of the bottle and close it hermetically with a cork. Take the water sample to the laboratory for quality control as soon as possible.
To make it quick and convenient for you to get to the nearest laboratory and be sure that the results of the analysis are correct, please contact an accredited laboratory, for example, the SES laboratory.
Attention!
- The selected sample must be delivered to the laboratory within 4 hours, and if prompt delivery is not possible, keep the water sample in the refrigerator at 2..6 ° C, but not more than 48 hours.
- It is advisable to clarify in advance the time for receiving samples at the laboratory of your choice and the terms of payment for water analysis.
Sampling for microbiological analysis is carried out in a sterile container, which must be taken in advance in the laboratory. In this case, it is necessary to take a water sample under conditions that exclude extraneous microbiological contamination, for example, from the air.


