Anion soap. Harm from pav
Superficially - active substances (Surfactants) are part of almost every shampoo with rare exceptions. The main purpose of surfactants - foaming.
The harm of surfactants is that they are extremely active chemical compounds. Their effects on humans are not fully understood, but it has been scientifically proven that surfactants are able to penetrate the body and accumulate on cell membranes. When a certain concentration of surfactants is reached, they cause changes in the biochemical processes that take place in the cells of our body and provoke dysfunction and integrity of the cells. Harmful substances in cosmetics today are not uncommon.
All surfactants used by modern industry can be divided into several groups: anionic, cationic, non-ionic and ampholytic.
Anionic surfactants have the most aggressive effect. They can cause allergic reactions, immunity disorders, disrupt the function of the kidneys, liver, cardiovascular system. There is evidence that they also have a carcinogenic effect.
Among the surfactants found in hair shampoos, the most dangerous are Ammonium Laureth Sulfate, Sodium Lauryl Sulfate, Ammonium Lauryl Sulfate and Sodium Laureth Sulfate.
These compounds simultaneously with the removal of dirt violate the structure of the hair, making them brittle, naughty, dull. Surfactants cause irritation of the scalp, itching, dandruff. Regular use of a shampoo with a surfactant content can cause hair loss.
Thus, all the magic properties of the shampoo, promised colorful advertisements, are leveled by the presence in its composition of harmful chemical compounds.
The amount of surfactant in the composition of the shampoo can be very large. On the label, all ingredients are listed in descending order. At the top of the list, as a rule, water flows, followed by frothers, i.e. Surfactant.
The list of surfactants and their harmful effects on the human body can be continued indefinitely. Currently, credible manufacturers are trying to exclude harmful surfactants from the shampoo. Consequently, the consumer has a choice, you just need to carefully study the composition of the shampoo.
Of course, natural shampoos that do not contain harmful surfactants, foam less, but their cleaning ability is no worse. And most importantly, such products are safe for your health.
- How to avoid toxins in the care of teeth, skin and hair
- Impeccable pedicure: 8 tips
- Cosmetics from oil: closer than it seems
Compounds that are in water solutions depending on the pH of the medium, they ionize differently and act - in an acidic solution they show the properties of cationic surfactants, and in alkaline solutions - anionic surfactants. .
General information
At pH values, called the isoelectric point, amphoteric surfactant molecules exist in the form of dipolar balanced ions. At the isoelectric point, the charges are equal, and the amphoteric surfactant molecule is a zwitterion. The most common are cationic and anionic-oriented zwitterionic amphoteric surfactants. http://www.ngpedia.ru/id407917p1.html Primary, secondary or tertiary amino groups, pyridine or imidazoline groups usually serve as cationic groups. Instead of nitrogen, there can be sulfur, phosphorus, arsenic, etc. Carboxyl, sulphonate, sulfoester and phosphate groups are used as anionic groups. In general, amphoteric surfactants can be represented by the formula:
K - -R-O +, where R is a hydrocarbon radical, usually C 9 -C 19; O + is the main group; K - - acid group.
Classification
According to the chemical structure, ampholytic surfactants are divided into five main types. :
1.Alkylaminocarboxylic acids (AAAA):
- RNH (CH 2) nCOOH - the alkyl radical of an amine usually has a linear structure, the radical between the amine carboxyl groups sometimes has a branched character;
- alkylaminophenylcarboxylic acids - RNHC 6 H 4 COOH;
2.Alkylbetaine (AB) is the most common type of ampholytic surfactants:
- C- and N-alkyl betaines - RCH (N + (CH 3) 3) COO-RN + (CH 3) 2 CH 2 COO -;
- sulfobetaines - RC 6 H 4 CH 2 N + (CH 3) 2 CH 2 CH 2 OSO 3 -;
- sulfite betaine - RN + (CH 3) 2 CH 2 CH 2 OSO 2;
- phosphate betaines RN + (CH 3) 2 CH 2 CH (OH) CH 2 OPO 3;
- amidobetaines RCONH (CH 2) 3 N + (CH 3) 2 COO -;
- ethoxylated betaines RN + (CH 2 CH 2 O) CH 2 COO -.
3. Derivatives of alkyl imidazolines. In the structure of imidazoline ampholytic surfactants, anionic and cationic groups are approximately equivalent. By structure, they can be divided into two main classes - non-beta and beta:
here, R is a C 7 -C 17 hydrocarbon radical.
4. Alkylaminoalkanesulfonates and sulfates. The ionization constant of the acid group of these surfactants is much larger than the main one, therefore they are used in an alkaline medium. Depending on the ionization constants, salts can be distinguished:
- alkylaminoalkanesulfonate salt RN (R ") - R: -SO 3 M;
- alkylaminoalkanesulfate salt RN (R ") - R: - OSO 3 M;
- aromatic amino sulfonic acid derivatives RR’N-Ar-SO 3 M, where R and R "are long and short hydrocarbon radicals, R: is a short bivalent radical
5. Polymer ampholytic surfactants:
- natural (proteins, nucleic acids, etc.);
- stepwise condensation products of amines, formaldehyde, albumin, fatty acids;
- cellulose derivatives obtained by the introduction of carboxyl and ethanol aminoethyl groups;
- synthetic, in whose molecules the structural features of all the above amphoteric surfactant groups are combined.
Application
Amphoteric surfactants in combination with anionic surfactants improve foaming ability and increase the safety of formulations detergents, and when combined with cationic polymers, they enhance the positive effect of silicones and polymers on hair and skin. These derivatives are derived from natural raw materials, so they are quite expensive components. The most commonly used derivatives are betaine (cocamopropyl betaine). The chemical structure of ampholytic surfactants provides for the presence in their structure of many diverse functional groups and the possibility of constructing them in various combinations. Therefore, with the emergence of new directions in the application of surfactants and the study of the possibilities of obtaining drugs with given properties, ampholytic surfactants are the most promising.
Polar groups in anionic surfactants are usually carboxylate, sulfate, sulfonate and phosphate groups. In fig. 1 shows the structure of the molecules of the most common surfactants of this class.
Anionic surfactants are used in much larger volumes than other types of surfactants. According to a rough estimate, world production of surfactants is 10 million tons per year, of which 60% is accounted for by anionic surfactants.
Fig. 1. Structures of some typical anionic surfactants
The main reason for the popularity of these surfactants is simplicity and low cost of production. Anionic surfactants are part of most detergents, and surfactants with alkyl or alkylaryl groups containing 12-18 carbon atoms in the hydrophobic chain have the best detergent action.
The counter ions are usually Na +, K +, NH4 +, Ca 2+ and various protonated alkylamines. Sodium and potassium ions increase the solubility of surfactants in water, while calcium and magnesium ions increase the solubility of surfactants in the oil phase. Protonated amines and alkanolamines ensure the solubility of surfactants in both phases.
Soaps also make up a huge class of surfactants. They are produced by saponification of natural oils and fats. Commonly, soaps are alkali metal salts of carboxylic acids derived from animal fats or vegetable oils. Solid soaps, as a rule, contain fatty acids, which are obtained from tall oil, palm and coconut oils. When used in optimal conditions, soaps are ideal surfactants. Their main drawback is sensitivity to hard water, which determined the need to create synthetic surfactants. A very specific application is found in the lithium salt of a fatty acid, namely lithium hydroxystearate, which is used as the main component of lubricants.
Alkyl benzene sulphonates constitute a group of synthetic surfactants, which are commonly considered the main workhorses. They are widely used in household detergents, as well as in various industries. They are obtained in the process of sulfonation of alkyl benzenes. In large-scale synthesis, sulfur trioxide is most often used as a sulphating agent, but it is possible to use other substances such as sulfuric acid, oleum, chlorosulfonic, or amidosulfonic acids. In some cases, they are even more preferable. Industrial synthesis is carried out in a continuous process using a film apparatus with a free-flowing film. In the first stage of the process, pyrosulfonic acid is formed, which slowly and spontaneously reacts further, forming sulfonic acid.
Then the sulfonic acid is neutralized with caustic soda, with the formation of an alkyl benzene sulfonate salt. Due to the large volume of alkyl substituents, almost exclusively i-sulfonic acids are formed. In the above scheme, R is an alkyl group, usually containing 12 carbon atoms. Initially, branched alkyl benzenes were used as an intermediate in the synthesis of surfactants, but now they are almost completely replaced by linear derivatives, therefore such surfactants are called linear alkyl benzene sulfonates. The rejection of branched derivatives and their replacement with linear ones are mainly due to their faster biodegradation. Alkyl benzenes, in turn, are obtained by alkylation of benzene with n-alkenes or alkyl chlorides using HF or AICI3 as catalysts. The reaction produces a mixture of isomers with a phenyl group attached to one of the non-terminal positions in the alkyl chain.
Another type of sulfonate surfactants used in detergents are sulfonates of paraffins and α-olefins, the latter often referred to as AOS. In general cases, the resulting surfactants are complex mixtures of substances that differ in their physicochemical properties. Paraffin sulfonates, or n-alkane secondary sulfonates, are mainly produced in Europe. They are usually obtained by sulfooxidation of paraffin hydrocarbons with sulfur dioxide and oxygen when irradiated with ultraviolet light. In an older process, which, however, is still used, paraffin sulfonates are obtained by the sulfochlorination reaction. Both processes are radical reactions, and since secondary carbon atoms form more stable free radicals than primary carbon atoms, the sulfo group is introduced statistically to any non-terminal carbon atom of the alkane chain. A mixture of C 14 -C 17 hydrocarbons, sometimes called the “Euro-fraction”, is the most common hydrophobic raw material, and the final products in this case are represented by very complex mixtures of isomers and homologues.
Sulfonates of a-olefins are obtained by the reaction of linear a-olefins with tri-sulfur oxide; the result is a mixture of alkenesulfonates, 3- and 4-hydroxyalkanesulfonates and a certain amount of disulfonates and other substances. As a source of raw materials used mainly two olefinic fractions: C 12 -C 16 and C 16 -C 18. The ratio of alkene sulfonates to hydroxyalkanesulfonates is regulated to some extent by the ratio of the amounts of SO 3 and olefins introduced into the reaction mixture: the higher this ratio, the more alkene sulfonic acid is formed. The formation of hydroxyalkanesulfonic acid occurs through an intermediate cyclic sulton, which is then cleaved by alkali. Sulton is toxic, so it is important that its concentration in the final product is very low. The receiving scheme can be written as follows:
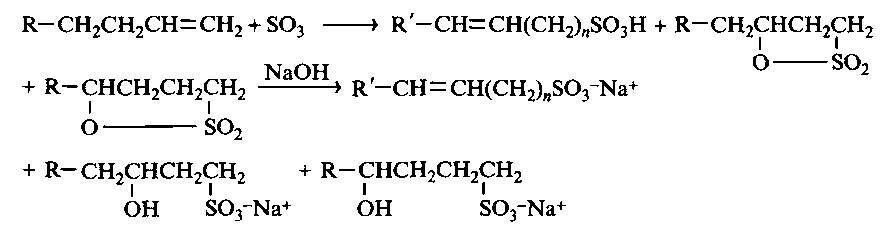
Sodium disulfosuccinate is an alkyl sulfonate surfactant widely used in studies of surface chemistry. This surfactant due to the bulk hydrophobic group is especially convenient for the production of microemulsions "water in oil".
Isethionate surfactants with the general formula R-COOC ^ C ^ SO ^ Na * are esters of fatty acids and isethionic acid salts. They belong to the mildest surfactants and are used in cosmetic formulations.
Sulfonate surfactants obtained by the sulfonation of lignin, petroleum fractions, alkylnaphthalenes or other cheap hydrocarbon fractions find widespread industrial use as dispersants, emulsifiers, demulsifiers, defoamers, wetting agents, etc.
Sulfonated sirthas and ethoxylated alcohols constitute another important group of anionic surfactants, which are widely used in detergents. These are sulfuric acid monoesters, in which the ester bond is very unstable and relatively easily broken at low pH as a result of autocatalytic hydrolysis. Linear and branched alcohols with carbon numbers from 8 to 16 are used as raw materials for this type of surfactant. When using linear alcohol with 12 carbon atoms, dodecyl ester of sulfuric acid is obtained, and after neutralizing with caustic soda sodium dodecyl sulfate is formed - the most important surfactant of this type. Ethoxylated alcohols, commonly used as intermediates, are aliphatic alcohols with two or three oxyethylene units. The process is similar to the above sulfonation. In industrial production, sulfur trioxide is used as a reagent,
and similar to sulfonation, the reaction proceeds through the stage of formation of pyrosulfate as an intermediate:
The synthesis of sulfate esters of ethoxylated alcohols is carried out in a similar manner. The reaction is usually accompanied by the formation of a significant amount of 1,4-dioxane. Since dioxane is toxic, it must be freed from distillation. Such surfactants are commonly referred to as ethoxylated alkyl sulfates. They have good foaming properties, low toxicity to the skin and eyes, and therefore are used in detergent compositions for dishes and shampoos.
Ethoxylated alcohols can be converted to carboxylates, i.e. ethoxylated alkyl carboxylates. Traditionally, this was done using sodium monochloroacetate:
The Williamson reaction usually proceeds with a small yield. Newer methods of synthesis are based on the oxidation of ethoxylated alcohols with oxygen or hydrogen peroxide in an alkaline medium using platinum or palladium as a catalyst. In this reaction, ethoxylates are converted in good yield, but oxidative degradation of the polyoxyethylene chain is also possible. Ethoxylated alkyl carboxylates are used in the manufacture of personal care products or as a co-surfactant in various liquid detergent formulations. Like ethoxylated alkyl sulfates, ethoxylated alkyl carboxylates are stable in very hard water. Both types of surfactants also have a good ability to disperse calcium soaps, which is very important for surfactants that are part of personal care products. The ability to disperse calcium soaps is usually expressed as the amount of surfactant that is required to disperse calcium soaps obtained from 100 g of sodium sodium in water with a hardness equivalent to 0.0333% CAS02.
The most important information about anionic surfactants
1. Anionic surfactants - the most common class of surfactants.
2. Usually anionic surfactants are incompatible with cationic surfactants.
3. They are sensitive to hard water, and the sensitivity decreases in the series carboxylates\u003e phosphates\u003e sulfates “sulfonates.
4. The introduction of a short polyoxyethylene chain between the anionic group and the hydrocarbon radical significantly increases the stability of anionic surfactants to salts.
5. The introduction of a short polyoxypropylene chain between the anionic group and the hydrocarbon radical increases the solubility of surfactants in organic media, but at the same time can lead to a decrease in the rate of biodegradation of surfactants.
6. Sulfate surfactants as a result of autocatalytic hydrolysis are rapidly hydrolyzed in acidic media. Other types of surfactants are stable in not too harsh conditions.
All commercial phosphate surfactants contain complex mono - and diesters of phosphoric acid, and the relative content of these components varies widely depending on the manufacturer. Since the physicochemical properties of alkyl phosphate surfactants depend on the ratio of different esters, alkyl phosphates from different manufacturers are less interchangeable than other types of surfactants. POCI 3 phosphorus oxychloride can be used as a phosphorylating agent for the production of alkyl phosphate surfactants. In this case, a mixture of mono- and diesters of phosphoric acid is also formed.


