What is a surfactant. Surfactants: General Information
In relation to the non-polar phase (gas, hydrocarbon, non-polar surface of a solid), there is a hydrocarbon radical that is pushed out of the polar medium. In an aqueous surfactant solution, an adsorption is formed at the interface with hydrocarbon radicals oriented to the side. As it is saturated (ions) surfactant, compacted in the surface layer, arranged perpendicular to the surface (normal orientation).
Depending on the state of the surfactant in the solution, conditionally distinguish between truly soluble (molecularly dispersed) and colloidal surfactants. The convention of such a division is that the same surfactant can belong to both groups depending on the conditions and chemical. nature (polarity) of the solvent. Both groups of surfactants are adsorbed on the phase boundaries, i.e. they are manifested in solutions, while the bulk properties associated with the onset of the colloidal (micellar) phase are manifested only by colloidal surfactants. These surfactant groups differ in the value of the dimensionless quantity, which is called. hydrophilic-lipophilic balance (HLB) and is determined by the ratio:
where is the affinity (free interaction energy) of the non-polar part of the surfactant to the hydrocarbon (b-dimensionless parameter depending on the nature of the surfactant, -freedom interaction energy per CH 2 group, v is the number of CH 2 groups in the hydrocarbon radical), a - affinity of the polar group k. For colloidal surfactants (b + or, where the indices m correspond to the minimum affinity values at which the colloidal properties of surfactants begin to manifest. The minimum number of carbon in the radical for different types of colloidal surfactants lies between 8 and 12, that is, colloidal surfactants have a rather large hydrocarbon radical. However, colloidal surfactants must also have true solubility in, i.e., the polarity of the hydrophilic group must also be sufficiently high. This corresponds to the condition:
![]()
In the beginning. 60s. 20 in. D. Davis developed the HLB scale with values from 0 to 40. Surfactants with lipophilic properties have low HLB values, with hydrophilic-high values. Each group is included in the surfactant is assigned a group number. When adding these numbers get HLB by the formula:
HLB = hydrophilic group numbers + 4-hydrophobic group numbers + 7.
Although the concept of HLB is quite formal, it allows you to determine the scope of the surfactant. So, for the formation of water / oil HLB is in the range of 3-6, oil / yes-8-16, for wetting agents-7-9, for funds-13-15.
Amphoteric (ampholytic) surfactants contain a hydrophilic radical and a hydrophobic part capable of being an acceptor or donor depending on the pH of the solution. Typically, these surfactants include one or more basic and acidic groups, and may also contain a non-ionic polyglycolic group. Depending on the pH value, they exhibit the properties of cation-active or anion-active surfactants. At some pH values, called , Surfactants exist in the form of zwitterions. The ionization constants of acidic and basic groups of truly soluble amphoteric surfactants are very low, however, cation-oriented and anion-oriented zwitterion are most common. As the cationic group, the primary, secondary or tertiary ammonium group is usually used, the residue or. In principle, instead of N m. B. S, P, As, etc. Anionic groups are carboxyl, sulphonate, sulfoether or phosphate groups.
By chemical structure and some similarity of properties ampholytic surfactants are divided into 5 DOS. groups: 1) alkylaminocarboxylic acids RNH (CH 2) n COOH; The alkyl radical is usually normal (straight-chain), but if it is located between the amine group and the carboxyl group, it sometimes has a branched character. Alkylamino-phenylcarboxylic acids RNHC 6 H 4 COOH; alkylaminocarboxylic acids with primary, secondary or tertiary amino group RCH (NH 2) COOH, RCH (NHR) COOH, R (CH 3) NCH 2 COOH; with an intermediate hydroxyl, ester, ester, amide or sulfoamide group; substances with two or more amino and amido groups, with several amino and hydroxyl groups.
2) Alkyl betaines are the most important group of zwitterionic surfactants. They can be divided into 5 DOS. groups: a) alkyl betaines-C-alkyl betaines RCH COO - and N-alkyl betaines RN + (CH 3) 2 CH 2 COO -; b) sulphite-, sul-foo-, sulphate- and phosphate betaine RN + (CH 3) 2 CH 2 CH 2 RN + (CH 3) 2 CH 2 CH 2, RC 6 H 4 CH 2 N + (CH 3) 2 CH 2 CH 2 RN + (CH 3) 2 CH 2 CH (OH) CH 2 OP; c) amidobetaines RCONH (CH 2) 3 N + (CH 3) 2 COO -; d) ethoxylated RN + [(C 2 H 4 O) p H] [(C 2 H 4 O) g H] CH 2 COO -; e) other zwitterionic surfactants.
3) Derivatives of alkyl imidazolines, in which the anionic and cationic groups have approximately the same ionization constants (formulas VII and VIII), where R is alkyl C 7 -C 17, R "-H, Na, CH 2 COOM (M-metal). By betaine surfactants, including a carboxy, sulfo, sulphate or sulfoester group, are distinguished in the structure and methods of synthesis [formula IX; R "= (CH 2) n COO -, (CH 2) 3, CH 2 CH (OH) CH 2] and other ("non-betaine") imidazoline surfactants [formula X; R "= CH 2 COONa, (CH 2) 2 N (CH 2 COOH) 2, (CH 2) 2 N = = CHC 6 H 4 SO 3 H, (CH 2) 2 OSO 3 H]. The balance of the ionized groups ensures these compounds have good colloid-chemical and sanitary-hygienic properties.
4) Alkylaminoalkanesulfonates and sulfates (AAAC 1 and AAAC 2 respectively). Anionic landmark. substances easily transform into the zwitterionic form, which allows them to be isolated in pure form. The ionization constant of the acid group is much larger than the main one, so they are used in an alkaline medium. However, in the case of several main groups, and in the presence of other hydrophilic groups near the acid group, these substances are similar in properties and applications to ampholytic surfactants and have a bactericidal effect. Depending on the ionization constants, it is possible to distinguish AAAC 1 RN (R ") - R: -SO 3 M, AAAC 2 RN (R") - R: - OSO 3 M, derivatives of aromatic amino sulfonic acids RR "N-Ar-SO 3 M, aminosulfonates with N in heterocycles (formula XI), aminophosphates, aminophosphonates and other amino-containing compounds of the type RR "R: P (O) (OH) 2, RR" R "" OP (O) (OH) 2, where R and R " -long and short hydrocarbon radicals, R: -short divalent radical; Comm. RN (CH 2 CH 2 SO 3 Na) 2. Their difference is a good ability to disperse calcium and resistance to.
5) Polymeric ampholytic surfactants: natural (proteins, nucleic acids, etc.); modified natural (oligomeric hydrolysates, sulfatir. chitin); staged foods, fatty acids; derivatives obtained by the introduction of carboxyl and diethanolaminoethyl groups; synthetic, which combine the structural features of all the above groups of amphoteric surfactants (see, for example, formulas XII-XVI).
The use of surfactants.The global production of surfactants is 2-3 kg per capita per year. Approximately 50% of the surfactants produced are used for (detergents and cleaning products, cosmetics), the rest in industry and with. x-ve. Simultaneously with the annual growth of surfactant production, the ratio between their use in everyday life and industry is changing in favor of industry.
The use of surfactants is determined by them, the structure of the adsorption layers and the bulk properties of the solutions. Surfactants of both groups (truly soluble and colloidal) are used as dispersants for drilling hard rocks (hardness reducer), for improving, lowering and wear, oil recovery rates, etc. Dr. an important aspect of the use of surfactants - the formation and destruction,. Surfactants are widely used for regulation and stability with a liquid dispersion medium (aqueous and organic). Micellar systems formed by surfactants in both aqueous and non-aqueous media are widely used, for which it is not the surface activity of surfactants that is important and not the properties of their adsorbers. layers, and bulk properties: pronounced anomalies with an increase in surfactant until the formation, for example in an aqueous medium, crystallization. structures of solid or solid-shaped structures (in based on petroleum oils).

Surfactants are used in more than 100 sectors of the economy. Most of the surfactants produced are used in the composition of the Wed-in, in the production of textiles and products based on synthetic. and prir. fibers. The major consumers of surfactants include oil, chemical. industry, industry builds. materials and a number of others. The most important surfactant applications are:
Drilling with clay solutions and reversible water / oil. To regulate the aggregative stability and rheological characteristics of the solutions, high-molecular-weight water-soluble surfactants, polyacrylamide, etc. are used, and calcium sources are introduced into it. and synthetic fatty acids (C 16 -C 18 and above), alkyl aromatic. alkylamines, alkylamido amines, alkyl imidazolines;
Enhance oil recovery by micellar flooding (ethoxylated and alkylaromatic sulfonates);
Antioxidant, extreme pressure and other additives in the production of the miner. oils (synthetic soaps. fatty acids, petroleum, hydroxyethyl. alcohols) and plastic. lubricants (derivatives, arylamines, alkyl and aryl phosphates);
Regulation with iron and manganese (soaps of natural and synthetic fatty acids, higher aliphatic amines), rare (alkylaronic and alkylphosphonic acids, alkyl aromatic sulfonates);
Emulsion, production and other vinyl (carboxymethylcellulose, poly, synthetic. Fatty acids, alkyl sulfates, and alkylphenols);
Chemical production fibers (hydroxyethyl. and amides, and, higher and acids);
Mechanical restoration
Course work
Chemistry of surfactants
Adsorption of surfactants at interphase boundaries
Surfactants are characterized by a pronounced ability to adsorb on surfaces and on interfaces. The term "interphase boundary" is usually referred to the boundary between two immiscible phases, the term "surface" indicates that one of the phases is a gas, as a rule, air. Thus, there are five different interphase boundaries:
solid - steam
solid - liquid
solid - solid
liquid - liquid
The driving force behind the adsorption of surfactants on surfaces and at interphase boundaries is the reduction of the free energy of the interface. The interfacial free energy per unit area is equal to the work that needs to be done to increase the surface. Instead of the term "interfacial free energy per unit area", the term interfacial tension is often used. Thus, the surface tension of water is equivalent to the specific free energy of the boundary between water and air. If the surface is coated with surfactant molecules, the surface tension decreases. The tighter the packing of surfactant molecules on the surface, the greater the decrease in surface tension.
Surfactants can adsorb to any of the five phase boundaries listed above. Examples of various interphase boundaries and systems in which such boundaries play an important role are given in Table. one.
In many composite products there are several types of interfaces present at the same time. Examples include water based inks and dyes for paper. From the point of view of colloid chemistry, they are extremely complex systems in which there is a solid-liquid interface and a liquid-liquid interface. In addition, at the stage of application of these systems, foaming is often observed with the appearance of a liquid-gas interface. All interphase boundaries at the same time are stabilized by surfactants and the total interfacial surface is huge. The interfacial boundaries of oil-water and solid water, existing in one liter of paint, can cover the surface of several football fields.
The desire of surfactants to accumulate at interphase boundaries is their fundamental property. In principle, the stronger this ability, the higher the effectiveness of surfactants. The degree of surfactant concentration on the surface depends on the structure of their molecules and on the nature of the contacting phases. Therefore, there is no universal effective surfactant suitable for any systems. The selection of a suitable surfactant is determined by the functions that it must perform in this system. An effective surfactant should have low solubility in liquid phases. Some surfactants are insoluble in water and in non-polar liquids and are localized only at interphase boundaries. It is difficult to work with such substances, but they very effectively reduce the interfacial tension.
Surfactant is able to reduce surface or interfacial tension to a certain limit. Usually this limit is reached when micelle formation begins in the solution. From the data given in table. 2, it is clear how effectively surfactants reduce surface and interfacial tension. The indicated values of surface and interfacial tension are typical for ordinary soft liquid detergents. Specially selecting surfactants, one can achieve ultra-low interfacial tension, i.e. values of the order of 10 -3 mN / m or below. As an example of systems with ultralow interfacial tension, one can cite a three-phase system consisting of a microemulsion in equilibrium with an excess of the aqueous and oil phases. Microemulsions are of interest for enhanced oil recovery.
Table 2. Typical values of surface and interfacial tension
Aggregation of surfactants in solution
As noted above, the fundamental property of surfactants is the ability to adsorb at interphase boundaries. Another important property of surfactants is that their molecules are prone to the formation of aggregates - the so-called micelles. Free or unassociated surfactant molecules are often referred to as monomers in the literature, although this term is unsuccessful because it is primarily used to refer to blocks that form polymer molecules. Micelle formation can be considered as an alternative mechanism of adsorption at interphase boundaries, leading to the elimination of the contact of hydrophobic groups with water, as a result of which the free energy of the system decreases. This is an extremely important phenomenon, because the properties of surfactants are determined by the form in which they are micellar or molecular - they are present in the system. Only molecularly dissolved surfactants reduce surface and interfacial tension; in addition, dynamic phenomena are governed by the concentration of the truly dissolved surfactant.
Micelles can be considered as reservoirs of molecular surfactants. Depending on the size and structure of the surfactant molecule, the rate of exchange of surfactant molecules between the micelle and the solution can vary in size within several orders of magnitude.
In water, micelles appear already at very low concentrations. The concentration at which micelles begin to occur is called the critical concentration of micelle formation; This is one of the most important characteristics of surfactants. Thus, CCM = 1 mM means that the concentration of the molecularly dissolved surfactant will never exceed this value regardless of the amount of surfactant introduced into the solution.
Amphiphilic properties of surfactant molecules
The term "amphiphil" is often used interchangeably with surfactants. The term is derived from the Greek word amphi, meaning "both." Its use is due to the fact that the molecules of all surfactants consist of at least two parts, one of which is soluble in the liquid, and the second is insoluble. If the liquid is water, they speak of hydrophilic and hydrophobic parts of the molecule, respectively. The hydrophilic part is usually called the polar group or “head”, and the hydrophobic part is called the radical or “tail.”
In a micelle, the hydrophobic groups are located inside the aggregate, and the polar groups are directed toward the solvent. Therefore, the micelle is a polar aggregate, well soluble in water, and itself does not have a noticeable surface activity. When a surfactant is adsorbed from an aqueous solution on a hydrophobic surface, the surfactant molecule is usually oriented by the hydrophobic part to the surface, and the polar group to water. The interfacial surface at the same time becomes hydrophilic, as a result the interfacial tension decreases. Adsorption on hydrophilic surfaces often leads to the appearance of more complex aggregates of surfactant molecules.
The hydrophobic part of the surfactant molecule can be linear or branched. The polar group, as a rule, but not always attached to the end of the alkyl chain, which usually contains from 8 to 18 carbon atoms. The degree of branching of the chain, the position of the polar group and the length of the chain are the most important parameters determining the physicochemical properties of surfactants.
The polar group of the surfactant can be ionic or non-ionic, which largely determines the properties of the surfactant. This allows the classification of surfactants to ionic and non-ionic. The size of the polar group of non-ionic surfactants can vary widely. In ionic surfactants, the size of the polar group is more or less constant. It should be emphasized that the physicochemical properties of surfactants in a solution are determined by the ratio of the sizes of the hydrophobic and polar groups, and not by their absolute sizes.
Fig. 1. Schematic representation of the surfactant molecule
Usually surfactant contains only one polar group. Recently there has been a noticeable interest in dimeric surfactants containing two hydrophobic tails and two polar groups connected by a short bridge. Such substances have not yet found practical application, but they have interesting physicochemical properties. These surfactants effectively reduce surface tension and have very low CMC values. For comparison, the CMC of a conventional cationic surfactant, dodecyltrimethylammonium bromide, is 16 mM, and the CMC of the corresponding dimeric surfactant with two carbon atoms in the bridge connecting the monomers is 0.9 mM. The difference in the CMC values of ordinary and dimeric surfactants can be of great practical importance. A typical example of a dimeric surfactant is shown in Fig. 2
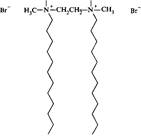
Fig. 2. Dimeric surfactant
Inefficient surfactants that can adsorb to the surface but do not form micelles are used as additives in many surfactant compositions. Such surfactants are classified as hydrotropic substances; they destroy the ordered packaging of conventional surfactants. For example, the introduction of a hydrotropic substance can prevent the formation of highly viscous liquid-crystalline phases, the occurrence of which often creates significant difficulties in the preparation of surfactant compositions. Xylene sulphonate and cumene sulphonate are typical representatives of hydrotropic substances used in the composition of many detergents. Short-chained alkylphosphates are widely used as hydrotropes in compositions based on long-chain ethoxylated alcohols.
Natural Surfactants
Naturally, surfactants of natural origin primarily include polar lipids. They are widely distributed in living organisms. In biological systems, surfactants perform essentially the same functions as synthetic surfactants in technical systems.
For example, they help the body overcome the problem of solubility of poorly soluble substances, are emulsifiers and dispersants, as well as surface modifiers, etc. Many interesting examples can be given that characterize the role of surfactants in biological systems. So, bile salts are extremely effective solubilizers of hydrophobic blood components of a mixture
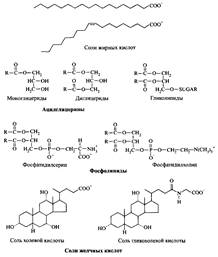
Fig. 3. Examples of polar lipids
phospholipids are packaged in ordered bilayers according to the type of surfactant liquid crystals and cell membranes consist of such structures. In fig. 3 shows examples of the most common polar lipids. Lecithin is a vivid example of a natural surfactant, which directly, without chemical procedures, is obtained from natural sources. Lecithin is extracted from phospholipid-rich sources.
Some microorganisms effectively produce natural surfactants. Both high molecular weight surfactants, such as lipopolysaccharides, and low molecular weight polar lipids can be obtained in good yields, especially if microorganisms are cultured on a water-insoluble substrate. In fig. 4 shows the structure of low molecular weight acylated carbohydrate - glycolipid based on trehalose, the high surface activity of which has already been proven. Such derivatives and some other surfactants produced by yeast have recently attracted much interest. Much effort has already been spent on improving existing fermentation processes and on developing new methods for cultivating microorganisms. Despite the progress made, the commercial use of these surfactants is still limited due to their high cost.
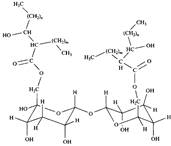
Fig. 4. Trehalose-based surfactant glycolipid obtained during fermentation
Petrochemistry and chemistry of vegetable oils as sources of raw materials for the production of surfactants
In recent years, there has been a tendency to use “green” surfactants, especially in everyday life. The term “natural surfactant” refers to the natural source of a substance. However, not a single surfactant used today in significant amounts can be considered natural in the full sense. With a few exceptions, all surfactants are produced in the process of organic synthesis, and often in very harsh conditions, when by-products are inevitably formed. For example, monoglycerides are widely distributed in nature, but surfactants, marketed as monoglycerides, are obtained during industrial hydrolysis of triglyceride oils at temperatures above 200 ° C, which leads to the formation of glycerol di-and tri-derivatives. Al-kilglucosides are extremely common in living organisms, but surfactants of this class, often referred to as APG, are produced using multi-step chemical processes, and they can certainly not be considered natural.
In order to properly assess the origin of surfactants, it is useful to divide them into two classes depending on the raw materials from which they are derived: oleochemical and petrochemical surfactants. Oleochemical surfactants are produced from renewable raw materials, usually from vegetable oils. Petrochemical surfactants are produced from small "building blocks", such as ethylene, obtained by cracking oil. Often the raw material for surfactants at the same time are vegetable oils and petrochemicals. Ethoxylated fatty acids are one of numerous examples.
Sometimes oleochemical and petrochemical methods of processing lead to the production of identical products. For example, normal alcohols with C10-C14 hydrocarbon radicals, commonly used for the introduction of hydrophobic groups in the synthesis of non-ionic surfactants and anionic surfactants, are obtained either by hydrogenating the methyl esters of the corresponding fatty acids or by catalyzing ethylene with triethyl aluminum. In both cases, unbranched aliphatic alcohols are obtained, which differ slightly in the composition of homologues, since it is determined by the distillation process. Both production methods are widespread.
The production of surfactants using vegetable oils as a raw material does not always ensure the production of less toxic and less environmentally harmful surfactants than petrochemical productions. However, taking into account the carbon cycle, chemical production based on renewable raw materials is always more preferable.
Alcohols with long linear hydrophobic radicals are often called fatty alcohols, regardless of how they are prepared. Alcohols with branched hydrocarbon radicals are also of great importance as a raw material for the production of surfactants. They are produced only by synthetic methods; Among them, the most common is the so-called oxoprocess, in which, as a result of the reaction of olefin with carbon monoxide and hydrogen, an aldehyde is obtained, which is then reduced to alcohol during catalytic hydrogenation. The result is a mixture of branched and normal alcohols, the ratio between which can be controlled to some extent by the selection of the catalyst and the reaction conditions. Commercial “oxo alcohols” are mixtures of normal and branched alcohols with a certain length of alkyl chains. Various methods for the preparation of primary long-chain alcohols are schematically shown in fig. five.
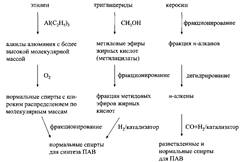
Fig. 5. Various ways of obtaining primary alcohols as raw materials for the production of surfactants.
From left to right: ethylene polymerization by the Cycloranate reaction; reduction of fatty acid methyl esters; hydroformylation of higher olefins.
Surfactant classification by polar groups
The first classification of surfactants is based on the charge of the polar group. It is generally accepted to divide surfactants into anionic, cationic, non-ionic, and zwitterionic. The surfactant molecules in the last group, under normal conditions, contain both charges: anionic and cationic. In the literature, they are often referred to as “amphoteric” surfactants, but this term is not always correct and should not be used as a synonym for the term “zwitterionic” surfactant. Amphoteric surfactant is a substance that, depending on the pH of the solution, can be cationic, zwitterionic or anionic. A prime example of amphoteric organic substances is simple amino acids. Most of the so-called Zvit-ter-ionic surfactants have similar properties. However, some zwitterionic surfactants retain one of the charges in a wide pH range, for example, compounds that include the cationic quaternary ammonium group. Thus, a surfactant containing carboxylate and quaternary ammonium groups will be zwitterionic up to very low pH values, but will not be amphoteric.
Most ionic surfactants are monovalent, but there are also important representatives of bivalent anionic surfactants. The physico-chemical properties of ionic surfactants are influenced by the nature of the counterion. In most cases, the anionic surfactant acts as a counter-ion sodium ion, while other cations, such as lithium ions, potassium, calcium or protonated amines, are used as such only for special purposes. Counterions for cationic surfactants are usually halide ions or methyl sulfate ions.
Hydrophobic surfactant groups are usually represented by hydrocarbon radicals, as well as polydimethylsiloxane or fluorocarbon groups. Surfactants of the latter two types are particularly effective in non-aqueous media.
For a small number of surfactants, there is some uncertainty in the classification. For example, surfactants containing amine oxides are sometimes referred to as zwitterionic, sometimes as cationic, and even as non-ionic surfactants. The charge of molecules of these substances depends on the pH of the aqueous phase; it is possible to sieve that, in the neutral state, they carry anionic and cationic charges or are non-ionic dipole molecules. Ethoxylated fatty amines containing the nitrogen atom of the amino group and the polyoxyethylene chain may be included in the class of cationic or non-ionic surfactants. The non-ionic character of such surfactants prevails in the case of very long polyoxyethylene chains, while with short or medium length polyoxyethylene chains, the physicochemical properties usually correspond to cationic surfactants. Surfactants containing an anionic group in the molecule, such as the sulphate, phosphate or carboxylate, and polyoxyethylene chains, are also quite common. Such surfactants, such as sulfoesters and others, usually contain short polyoxyethylene chains, and therefore are always considered as anionic surfactants.
Anionic Surfactants
Polar groups in anionic surfactants are usually carboxylate, sulfate, sulfonate and phosphate groups. In fig. 6 shows the structures of the molecules of the most common surfactants of this class.
Anionic surfactants are used in much larger volumes than other types of surfactants. According to a rough estimate, world production of surfactants is 10 million tons per year, of which 60% is accounted for by anionic surfactants.
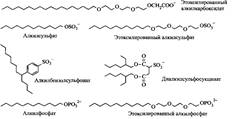
Fig. 6. Structures of some typical anionic surfactants
The main reason for the popularity of these surfactants is simplicity and low cost of production. Anionic surfactants are part of most detergents, and surfactants with alkyl or alkylaryl groups containing 12-18 carbon atoms in the hydrophobic chain have the best detergent action.
The counter ions are usually Na +, K +, NH4 +, Ca 2+ and various protonated alkylamines. Sodium and potassium ions increase the solubility of surfactants in water, while calcium and magnesium ions increase the solubility of surfactants in the oil phase. Protonated amines and alkanolamines ensure the solubility of surfactants in both phases.
Soaps also make up a huge class of surfactants. They are produced by saponification of natural oils and fats. Commonly, soaps are alkali metal salts of carboxylic acids derived from animal fats or vegetable oils. Solid soaps, as a rule, contain fatty acids, which are obtained from tall oil, palm and coconut oils. When used in optimal conditions, soaps are ideal surfactants. Their main drawback is sensitivity to hard water, which determined the need to create synthetic surfactants. A very specific application is found in the lithium salt of a fatty acid, namely lithium hydroxystearate, which is used as the main component of lubricants.
Alkyl benzene sulphonates constitute a group of synthetic surfactants, which are commonly considered the main workhorses. They are widely used in household detergents, as well as in various industries. They are obtained in the process of sulfonation of alkyl benzenes. In large-scale synthesis, sulfur trioxide is most often used as a sulphating agent, but it is possible to use other substances such as sulfuric acid, oleum, chlorosulfonic, or amidosulfonic acids. In some cases, they are even more preferable. Industrial synthesis is carried out in a continuous process using a film apparatus with a free-flowing film. In the first stage of the process, pyrosulfonic acid is formed, which slowly and spontaneously reacts further, forming sulfonic acid.
Then the sulfonic acid is neutralized with caustic soda, with the formation of an alkyl benzene sulfonate salt. Due to the large volume of alkyl substituents, almost exclusively i-sulfonic acids are formed. In the above scheme, R is an alkyl group, usually containing 12 carbon atoms. Initially, branched alkyl benzenes were used as an intermediate in the synthesis of surfactants, but now they are almost completely replaced by linear derivatives, therefore such surfactants are called linear alkyl benzene sulfonates. The rejection of branched derivatives and their replacement with linear ones are mainly due to their faster biodegradation. Alkyl benzenes, in turn, are obtained by alkylation of benzene with n-alkenes or alkyl chlorides using HF or AICI3 as catalysts. The reaction produces a mixture of isomers with a phenyl group attached to one of the non-terminal positions in the alkyl chain.
Another type of sulfonate surfactants used in detergents are sulfonates of paraffins and α-olefins, the latter often referred to as AOS. In general cases, the resulting surfactants are complex mixtures of substances that differ in their physicochemical properties. Paraffin sulfonates, or n-alkane secondary sulfonates, are mainly produced in Europe. They are usually obtained by sulfooxidation of paraffin hydrocarbons with sulfur dioxide and oxygen when irradiated with ultraviolet light. In an older process, which, however, is still used, paraffin sulfonates are obtained by the sulfochlorination reaction. Both processes are radical reactions, and since secondary carbon atoms form more stable free radicals than primary carbon atoms, the sulfo group is introduced statistically to any non-terminal carbon atom of the alkane chain. A mixture of C14-C17 hydrocarbons, sometimes called the “Euro-fraction”, is the most common hydrophobic raw material, and the final products in this case are represented by very complex mixtures of isomers and homologues.
Sulfonates of a-olefins are obtained by the reaction of linear a-olefins with tri-sulfur oxide; the result is a mixture of alkenesulfonates, 3- and 4-hydroxyalkanesulfonates and a certain amount of disulfonates and other substances. The feedstock used are mainly two olefinic fractions: C12-C16 and C16-C18. The ratio of alkene sulfonates to hydroxyalkanesulfonates is regulated to some extent by the ratio of the amounts of SO3 and olefins introduced into the reaction mixture: the higher this ratio, the more alkene sulfonic acid is formed. The formation of hydroxyalkanesulfonic acid occurs through an intermediate cyclic sulton, which is then cleaved by alkali. Sulton is toxic, so it is important that its concentration in the final product is very low. The receiving scheme can be written as follows:

Sodium disulfosuccinate is an alkyl sulfonate surfactant widely used in studies of surface chemistry. This surfactant due to the bulk hydrophobic group is especially convenient for the production of microemulsions "water in oil".
Isethionate surfactants with the general formula R-COOC ^ C ^ SO ^ Na * are esters of fatty acids and isethionic acid salts. They belong to the mildest surfactants and are used in cosmetic formulations.
Sulfonate surfactants obtained by the sulfonation of lignin, petroleum fractions, alkylnaphthalenes or other cheap hydrocarbon fractions find widespread industrial use as dispersants, emulsifiers, demulsifiers, defoamers, wetting agents, etc.
Sulfonated sirthas and ethoxylated alcohols constitute another important group of anionic surfactants, which are widely used in detergents. These are sulfuric acid monoesters, in which the ester bond is very unstable and relatively easily broken at low pH as a result of autocatalytic hydrolysis. Linear and branched alcohols with carbon numbers from 8 to 16 are used as raw materials for this type of surfactant. When using linear alcohol with 12 carbon atoms, dodecyl ester of sulfuric acid is obtained, and after neutralizing with caustic soda sodium dodecyl sulfate is formed - the most important surfactant of this type. Ethoxylated alcohols, commonly used as intermediates, are aliphatic alcohols with two or three oxyethylene units. The process is similar to the above sulfonation. In industrial production, sulfur trioxide is used as a reagent,
and similar to sulfonation, the reaction proceeds through the stage of formation of pyrosulfate as an intermediate:
The synthesis of sulfate esters of ethoxylated alcohols is carried out in a similar manner. The reaction is usually accompanied by the formation of a significant amount of 1,4-dioxane. Since dioxane is toxic, it must be freed from distillation. Such surfactants are commonly referred to as ethoxylated alkyl sulfates. They have good foaming properties, low toxicity to the skin and eyes, and therefore are used in detergent compositions for dishes and shampoos.
Ethoxylated alcohols can be converted to carboxylates, i.e. ethoxylated alkyl carboxylates. Traditionally, this was done using sodium monochloroacetate:
The Williamson reaction usually proceeds with a small yield. Newer methods of synthesis are based on the oxidation of ethoxylated alcohols with oxygen or hydrogen peroxide in an alkaline medium using platinum or palladium as a catalyst. In this reaction, ethoxylates are converted in good yield, but oxidative degradation of the polyoxyethylene chain is also possible. Ethoxylated alkyl carboxylates are used in the manufacture of personal care products or as a co-surfactant in various liquid detergent formulations. Like ethoxylated alkyl sulfates, ethoxylated alkyl carboxylates are stable in very hard water. Both types of surfactants also have a good ability to disperse calcium soaps, which is very important for surfactants that are part of personal care products. The ability to disperse calcium soaps is usually expressed as the amount of surfactant that is required to disperse calcium soaps obtained from 100 g of sodium sodium in water with a hardness equivalent to 0.0333% CAS02.
Phosphate-containing anionic surfactants, for example, alkyl phosphates or ethoxylated alkyl phosphates, are obtained by treating fatty alcohols or ethoxylated alcohols with a phosphorylating agent; usually, phosphorus pentoxide P4O10 is used for this. As a result of the reaction, a mixture of phosphoric acid mono- and diesters is obtained, the relative proportions of these substances being controlled by the ratio of the reactants and the amount of water in the reaction mixture:
The most important information about anionic surfactants
1. Anionic surfactants - the most common class of surfactants.
2. Usually anionic surfactants are incompatible with cationic surfactants.
3. They are sensitive to hard water, and the sensitivity decreases in the series carboxylates\u003e phosphates\u003e sulfates “sulfonates.
4. The introduction of a short polyoxyethylene chain between the anionic group and the hydrocarbon radical significantly increases the stability of anionic surfactants to salts.
5. The introduction of a short polyoxypropylene chain between the anionic group and the hydrocarbon radical increases the solubility of the surfactant in organic media, but at the same time can lead to a decrease in the rate of biodegradation of the surfactant.
6. Sulfate surfactants as a result of autocatalytic hydrolysis are rapidly hydrolyzed in acidic media. Other types of surfactants are stable in not too harsh conditions.
All commercial phosphate surfactants contain complex mono - and diesters of phosphoric acid, and the relative content of these components varies widely depending on the manufacturer. Since the physicochemical properties of alkyl phosphate surfactants depend on the ratio of different esters, alkyl phosphates from different manufacturers are less interchangeable than other types of surfactants. POCI3 phosphorus oxychloride can be used as a phosphorylating agent for the production of alkyl phosphate surfactants. In this case, a mixture of mono- and diesters of phosphoric acid is also formed.
Phosphate surfactants are used in the metalworking industry, where they are more suitable than other surfactants due to their anti-corrosion properties. They are also used as emulsifiers in the compositions used for plant protection. The most important information about anionic surfactants are summarized in Table. 3
Nonionic surfactants
Nonionic surfactants as the polar groups contain either polyester or polyhydroxy fragments. In the overwhelming majority of non-ionic surfactants, oxyethylene groups obtained by polymerization of ethylene oxide are added. Strictly speaking, the prefix "poly" is used incorrectly. The most common surfactants with the number of oxyethylene units in the polar chain are from 5 to 10, but some surfactants, such as dispersants, often contain longer oxyethylene chains. Ethoxylation is usually carried out in alkaline media. It is possible to ethoxylate any substance containing active hydrogen. Aliphatic alcohols, alkylphenols, fatty acids and aliphatic amines are usually used as starting materials for the production of oxyethylene nonionic surfactants. Esters, such as triglyceride oils, can be ethoxylated in a process in which alkaline hydrolysis of the ester bond proceeds in one reactor followed by ethoxylation of the acid and alcohol formed and their partial condensation. The ethoxylated castor oil used in animal feed is an important example of a surfactant based on triglycerides.
Examples of polyhydroxy surfactants are sucrose and sorbitol esters, alkyl glucosides and polyglycerides. The latter are actually a combination of surfactants, polyhydric alcohol derivatives and polyesters. Surfactants based on polyhydric alcohols can be ethoxylated. The best known examples are esters of fatty acids and sorbitol and the corresponding ethoxylated products. The five-membered ring of the sorbitan structure is formed when sorbitol is dehydrated during production. Sorbitan-based surfactants can be eaten, so they are widely used in the production of food and drugs. Acetylene glycols are surfactants containing an acetylene bond localized at the center and hydroxyl groups at adjacent carbon atoms. Such substances constitute a special group of hydroxyl surfactants used as antifoam agents, especially in the manufacture of coatings.
In fig. 7 shows the structures of the molecules of the most common non-ionic surfactants. As will be shown below, oxyethylene-based surfactants are represented by a wide range of compounds, which are much wider than surfactants of other classes. Fatty acid ethoxylates provide particularly complex mixtures with a high content of by-products. The most important type of non-ionic surfactant is ethoxylated aliphatic alcohols. They are used in the composition of liquid and powder detergents and are widely used in industry. In particular, they are used as stabilizers for oil-in-water emulsions. Ethoxylated alcohols can be considered resistant to hydrolysis in a wide pH range: from 3 to 11. They are slowly oxidized in air, and the oxidation products are more irritating to the skin than the original surfactants. In this book, ethoxylated fatty alcohols are designated as C t E p, where t is the number of carbon atoms in the alkyl chain of the alcohol, and n is the number of oxyethylene units in the surfactant molecule. Some common and most important properties of non-ionic surfactants are given in Table. four.
Table 4. The most important information about non-ionic surfactants
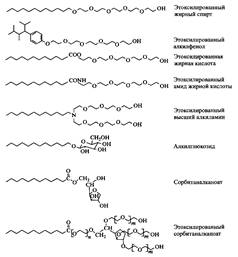
Fig. 7. Structures of some typical non-ionic surfactants
In ethoxylated surfactants, you can enter with great accuracy a given number of oxyethylene groups sewn to a specific hydrophobic residue, for example, to an aliphatic alcohol residue. However, the process ends with the formation of products with a wide distribution along the chain. If all the hydroxyl groups of the starting alcohol and the resulting glycol ethers have the same reactivity, a set of oligomers is obtained that obeys the Poisson distribution. Since the starting alcohol is somewhat less acidic than the glycol ethers formed, its deprotonation is less profitable, therefore, the probability of interaction with ethylene oxide is less.
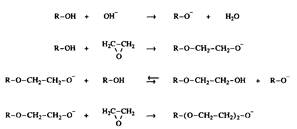
Fig. 8. Ethoxylation of fatty alcohols, catalyzed by bases
As a result, a significant amount of unreacted alcohol remains in the reaction mixture along with reaction products containing a larger number of oxyethylene groups. A lot of research efforts have been spent on developing ways to produce a product with a narrower distribution of homologs. Product distribution is also influenced by the choice of the ethoxylation catalyst. The use of alkaline earth metal hydroxides, such as Ba2 and Sr2, results in products with a much narrower distribution than with KOH, apparently due to the coordination mechanism. The use of Lewis acids, for example, SnCU and BF3, also allows the preparation of ethoxylated surfactants with a narrower distribution. However, ethoxylation in an acidic environment has a significant drawback: 1,4-dioxane is formed as a by-product in large quantities. In fig. 9 shows the distribution by homologues of an ethoxylated alcohol when KOH, Lewis acid and strontium hydroxide are used as catalysts.
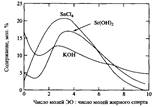
Fig. 9. Typical homolog distributions for the reaction products of a fatty alcohol with 4 mol of ethylene oxide in the presence of various catalysts.
Narrow homologous ethoxylates occupy a growing market share. The advantages of such ethoxylates are undoubted.
As can be seen from the table. 4, non-ionic surfactants containing polyoxyethylene chains have an inverse dependence of water solubility on temperature. With increasing temperature, the system is divided into two phases. The temperature at which this occurs is called the cloud point, as the solution becomes cloudy. Cloud point depends on the length of the hydrophobic part and the number of oxyethylene groups in the surfactant molecules and can be determined with great accuracy. In the production of polyoxyethylene surfactants, the determination of the cloud point is used to control the degree of ethoxylation. Since the turbidity of the system can vary with an increase in the surfactant concentration, in the standard test method, the cloud point is determined by heating a 1% aqueous solution of nonionic surfactants above the cloud point and then the temperature of the solution clears when the sample is slowly cooled. Surfactants with long polyoxyethylene chains can have a cloud point above 100 ° C. For such substances, the cloud point is determined in electrolyte solutions, since most salts lower the cloud point.
Ethoxylated triglycerides, for example castor oil ethoxylates, occupy stable positions in the market, they are often called semi-natural surfactants. In recent years, interest in ethoxylated methyl esters of fatty acids, which are obtained from the corresponding methyl ester, has been greatly increased through a process of ethoxylation using a special catalyst, for example, hydrotalcite. Methyl ether ethoxylates have a number of advantages over ethoxylated alcohols, since they are more soluble in water. Surfactants that combine high solubility in water with high surface activity are needed in a wide variety of compositions.
Ethoxylated alcohols, in which the terminal hydroxyl groups are replaced by a methyl or ethyl ester group, have their own market niche. Such "closed" end nonionic surfactants are produced by O-alkylation of ethoxylate by reaction with alkyl chloride or dialkyl sulfate or by hydrogenation of the corresponding acetal. Compared with ethoxylates of normal alcohols, surfactants with “closed” ends are more resistant to alkalis and oxidizing agents. They are also characterized by extremely low foaming capacity.
Cationic Surfactants
Most cationic surfactants contain a nitrogen atom that carries a positive charge, that is, they are amines or quaternary ammonium compounds. Amines exhibit surfactant properties only in the protonated state; therefore, they cannot be used at high pH. In contrast, quaternary ammonium compounds are insensitive to changes in pH. Amines are also more sensitive to the action of multiply charged anions. As already mentioned, ethoxylated amines have the properties of non-ionic and cationic surfactants, and the longer the oxyethylene chain, the more the non-ionic surfactants are expressed in such compounds.
In fig. 10. Some typical cationic surfactants are listed. Quaternary ammonium compounds with ester groups represent a new class of environmentally friendly surfactants, displacing the corresponding di-alkyl derivatives in the process of softening of tissues.
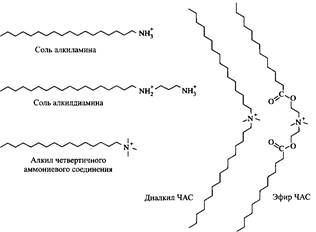
Fig. 10. Structures of some typical cationic surfactants.
Synthesis of non-ether quaternary ammonium surfactants passes through the formation of nitrile compounds. The fatty acid reacts with ammonia at elevated temperature to give the corresponding nitrile. This reaction goes through a step of forming an amide intermediate. Nitrile is then hydrogenated to the primary amine in the presence of a catalyst:
Secondary amines are obtained either directly from the nitrile, or in two stages from the primary amine. In a one-step process, which seems to go through the formation of an intermediate imine, ammonia is constantly removed from the reaction mixture to facilitate the formation of a secondary amine:
Primary amines are converted to long-chain 1,3-diamines by cyanoethylation:
Primary or secondary long chain amines can be methylated and converted to tertiary amines, for example, by reaction with formaldehyde under reducing conditions:
Ethylene oxide can also be used as an alkylating agent to convert primary and secondary amines to tertiary amines of the type R-CH2N2 and 2NCH 2 CH 2 OH.
Quaternary ammonium compounds are usually obtained from tertiary amines by reaction with a suitable alkylating agent, for example with methyl chloride or methyl bromide or dimethyl sulfate, and the choice of reagent is determined by the counterion surfactant:
Quaternary ammonium surfactants containing ester groups are obtained by esterification of the fatty acid with an amino alcohol followed by N-alkylation, as described above. As an example, the reaction of triethanolamine, taken as the amino alcohol, and dimethyl sulfate as the methylating agent is given:

Sulfoxonium surfactants are obtained by oxidation of a sulfonate salt with hydrogen peroxide. The industrial use of non-nitrogen cationic surfactants is small, since these substances rarely have advantages over cheaper nitrogen-containing surfactants. Phosphonium surfactants with one sufficiently long alkyl chain and three methyl groups have been used as biocides.
Most surfaces — metals, minerals, plastics, fibers, cell membranes, etc. — are negatively charged. The main use of cationic surfactants is associated with their ability to adsorb on negatively charged surfaces. Some examples are given in table. 5, and the most important characteristics of cationic surfactants - in Table. 6
Table 5. The use of cationic surfactants due to their adsorption on surfaces
Table 6. The main characteristics of cationic surfactants
Zwitterionic Surfactants
The zwitterionic surfactants contain two oppositely charged groups in the molecules. The positive charge is almost always provided by the ammonium group, and the negatively charged groups can be different; most often the negative charge is provided by the carboxylate ion. Such surfactants are often referred to as amphoteric, but, as noted above, these terms are not identical. The charges of an amphoteric surfactant change depending on the pH, while the transition from acidic to alkaline pH changes the type of surfactant from cationic via zwitterionic to anionic. Neither acidic nor basic groups carry a constant charge, and the zwitterion such a surfactant becomes only in a certain pH range.
A change in charge with a change in the pH of an amphoteric surfactant naturally affects its properties such as foaming and wetting abilities, detergency, that is, the main properties of surfactants are pH dependent. At the isoelectric point, the physicochemical properties of such surfactants are similar to those of non-ionic surfactants. Below and above the isoelectric point, there is a gradual shift to the cationic or anionic nature of the surfactant, respectively. Surfactants with sulphate or sulphonate groups providing a negative charge of molecules remain zwitterionic to very low pH values due to very low pK values of monoalkyl sulfuric and alkylsulfonic acids.
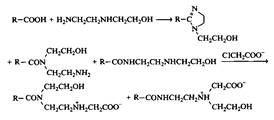
Typical representatives of zwitterionic surfactants are N-alkyl derivatives of simple amino acids, betaine 2 (NCH 2 COOH), aminopropionic acid). Such surfactants are obtained not from amino acids, but by the reaction of long-chain amines with sodium chloroacetate or with acrylic acid derivatives, with the formation of structures with one or two carbon atoms, respectively, between the nitrogen and carboxylate group. As an example, the following is the scheme of the reaction for obtaining a typical surfactant - a derivative of betaine from alkyldimethyl-amine and sodium monochloroacetate:

Amidobetaine derivatives are prepared analogously, starting from amidoamine.
Another type of zwitterionic surfactants, commonly called imidazolines, are synthesized by the reaction of a fatty acid with aminoethylethanolamine followed by treatment with chloroacetate. The nomenclature of this type of surfactant turned out to be somewhat confusing, since it was believed that the final product contained an imidazole ring, but later it was found that the five-membered ring was broken in the second stage of the synthesis. A typical reaction sequence is:
Civiton-ionic surfactants are characterized by very good dermatological properties, they do not irritate the eyes and therefore are often used in shampoos and cosmetics. Since the total charge of molecules of such surfactants is zero, they, like non-ionic surfactants, do not lose their properties in compositions with a high content of electrolyte. Traditionally, zwitterionic surfactants are used in the composition of alkaline cleaners. In fig. 11 gives examples of typical zwitterionic surfactants, and Table. 7 summarizes the basic information about surfactants of this class. As already mentioned, aminoxide surfactants, or, more correctly, tertiary amines N-oxides, are sometimes referred to as zwitterionic, sometimes as non-ionic, and sometimes as cationic surfactants. Formally, they have separated charges on the nitrogen and oxygen atoms and usually behave like non-electrolytes, but at low pH or in the presence of an anionic surfactant they accept a proton with the formation of a conjugate cationic acid. 1: 1-Valence salt is formed between an anionic surfactant and protonated aminoxide; such salts have high surface activity. Amine oxides are obtained by oxidation with hydrogen peroxide of the corresponding tertiary amine.
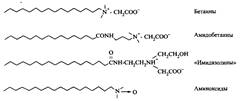
Fig. 11. Structures of some typical zwitterionic surfactants.
Dermatological effect of surfactants
The dermatological effect of surfactants causes serious problems and is the subject of many modern studies. The main dermatological problems in working conditions are associated with the contact of unprotected skin with surfactant solutions, which are used as a variety of cleaning agents, as well as fluids for cutting, oil emulsions for rolling, etc. Usually, the effect is reduced to irritation of the skin of varying severity, less often allergic reactions occur. Skin irritation is caused by direct exposure to the surfactant, and allergic reactions are activated by the by-products present in the surfactant compositions. A well-known example of a severe allergic reaction is the so-called “margarine disease”, discovered in the Netherlands in the 1960s. It was found that this disease causes a by-product found in a new surfactant that was added to margarine products to reduce splashing during frying. This surfactant allows you to save water droplets in a finely dispersed state. A substance with pronounced electrophilic properties turned out to be a sensitizing agent in the surfactant. When reacting with nucleophilic groups of proteins, their denaturation occurs, and the body perceives this substance as a foreign antigen. The surfactant used in the production of margarine, contained a significant amount of unreacted intermediate product, in which when released into the body, apparently, there was a cycle opening when interacting with amino or thiol groups of proteins.
The physiological effect of surfactants on the skin is investigated by various dermatological and biophysical methods, starting with the skin surface and the stratum corneum with its protective functions to a deeper layer of basal cells. Individual sensitivity or susceptibility of the skin is recorded by tactile sensations and experience. The surfactants that are mild to the skin include surfactants based on polyhydric alcohols, zwitterionic surfactants and isethionates. These surfactants are often used in cosmetics.
In the homologous series of surfactants, a maximum of irritating action is usually observed on the skin at a certain length of the hydrophobic radical. For example, in a comparative study of alkyl glucosides containing 8,10, 12,14 and 16 carbon atoms in the alkyl radicals, the maximum effect on the skin was found for C12-derivatives. The same maximum effect was found when studying the biocidal activity of surfactants. Apparently, these data reflect the fact that the biological reactions caused by the action of the surfactant on the mucosal membrane or bacterial surfaces, respectively, are due to the high surface activity and high concentration of the molecularly dissolved surfactant. Since an increase in the chain length of the hydrophobic “tail” of a surfactant leads to an increase in its surface activity and a decrease in CMC, i.e., a decrease in the molecularly dissolved surfactant, an extremum appears in the homologous series for a certain length of the hydrocarbon chain.
The ethoxylates of alcohols are relatively mild surfactants, but they are inferior in terms of the softness of the effect on the skin of nonionic surfactants based on polyatomic alcohols, for example alkyl glucosides. Recent studies have shown that the dermatological effects of alcohol ethoxylates are not caused by the surfactant itself, but by the products of its oxidation that occur during storage. All ethoxylated products were found to undergo auto-oxidation with the formation of hydroperoxides on methylene groups associated with ether oxygen in polyoxyethylene chains. Such hydroperoxides are not very stable, which makes them difficult to release. However, a hydroperoxide with a UN group at the second carbon atom of the hydrophobic "tail" is relatively stable and was isolated in an amount of ~ 1% after storage of the "-alkyl ethoxylate during the year. This substance has a strong irritant effect on the skin. Another oxidation product with a similar effect on the skin is an aldehyde, shown below. This aldehyde is unstable, and its further oxidation leads to the breaking of the polyoxyethylene chain and the formation of formaldehyde and other products. Both aldehydes irritate the skin and eyes:
To control the autoxidation of ethoxylated alcohols, it is useful to measure the change in the cloud point over time. In fig. 12 shows examples of such a test for C12E5 and C12E6. The figure shows that both nonionic surfactants show a sharp decrease in cloud point during storage at 40 ° C due to auto-oxidation.
Anionic surfactants, as a rule, have a greater effect on the skin than nonionic surfactants. So, sodium dodecyl sulfate, used in the composition of some personal care products, has a relatively high toxicity to the skin. Alkylsulfate ethers

Fig. 12. Dependence of cloud point on the storage time of 1% solutions of surfactants.
These nonionic surfactants did not contain homologous impurities. The measurements were carried out at two temperatures of sodium are softer surfactants compared with sodium alkyl sulfates, for this reason, ether derivatives are more often used in products for manual dishwashing. An important role for such agents is played by the good foam-forming properties of these surfactants. The best dermatological characteristics of alkyl sulfate esters compared to alkyl sulfates were the main reason for the interest in ethoxylates with a narrow distribution by homologues, which were discussed above. When sulfating such ethoxylates as an intermediate, the content of aggressive alkyl sulfates is noticeably lower than when using standard ethoxylates with a wide distribution by homologues.
The effect of surfactants on the skin is often determined using a test with a modified Dühring chamber. In fig. 13 shows typical results of such a test for sodium dodecyl sulfate, decyl glucoside, and mixtures thereof. The irritating effect on the skin decreases almost linearly with an increase in the mixture of glucoside surfactants. In other cases, even small supplements of mild surfactants can cause a very significant improvement in the dermatological properties of the compositions: such a synergistic effect may be associated with a strong decrease in the CMC composition due to the formation of mixed micelles. Some amphoteric surfactants greatly reduce skin irritation caused by contact with anionic surfactants, such as alkyl sulfate esters. The effect can be attributed to the protonation of carboxyl

Fig. 13. Test for the magnitude of the relative irritant.
The measurements were carried out using a modified Dühring chamber. With the permission of Wiley-VCH
groups of betaine surfactant, which turns into a cationic surfactant, followed by packing into mixed micelles with anionic surfactants. Thus, the gain in the energy of formation of mixed micelles leads to the protonation of the carboxyl group of the betaine surfactant already at pH values much larger than the pAG a.
The impact of surfactants on the environment
Despite the fact that concern about the effect of surfactants on the environment was legally registered more than 20 years ago, only recently this factor became the main requirement that determines the possibility of using surfactants in various compositions. A huge amount of surfactants used in everyday life and industry, goes into wastewater. The rate of biodegradation in the plant's septic tanks of wastewater determines the amount of surfactant entering the environment. Two parameters - the rate of biodegradation and the degree of toxicity in the aquatic environment - determine the potential impact of surfactants on the environment. The Organization for Economic Cooperation and Development has developed rules and guidelines for
Toxicity to water
Biodegradability
Bioaccumulation
Toxicity to water
Toxicity in the aquatic environment is measured on fish, daphnia or algae. Toxicity is expressed as LC50 or EC50, where LC and EC are lethal and effective surfactant concentrations, respectively. Values of surfactant concentrations below 1 mg / l, resulting in 96 hours of death of half of the individuals during the test on fish and algae and during the 48-hour test on daphnia, indicate the toxicity of the surfactant in the aquatic environment. Environmentally friendly surfactants should have appropriate values above 10 mg / l.
Biodegradability
Biodegradation is a process performed in nature by bacteria. As a result of a series of enzymatic reactions, the surfactant molecule eventually turns into carbon dioxide, water, and oxides of other elements. If the product is not subject to natural biodegradation, it is resistant and accumulates in the environment. The rate of biodegradation depends on the type of surfactant and ranges from 1-2 hours for fatty acids and 1-2 days for linear alkyl benzene sulfonates to several months for branched alkyl benzene sulfonates.
When determining the biodegradation of surfactants, it must be remembered that the rate depends on many factors: the concentration of surfactant, the pH of the solution and temperature. Temperature is particularly affected. The rate of decomposition of chemicals in factory sewage tanks varies almost five-fold in time depending on the time of year in Northern Europe.
Two criteria are important for determining biodegradation: primary decomposition and final products. The primary decomposition of the surfactant is associated with a loss of surface activity. For example, ester surfactants can quickly decompose into alcohol and acid, which do not have high surface activity. This criterion is of interest in special cases, for example, when deciding whether products will accumulate in the environment, causing foaming of water bodies.
From an ecological point of view, biodegradation end products are more important. There are many methods for testing biodegradability. Among them, the most popular was the modified Sturm test. In this test, the conversion of surfactant to carbon dioxide over time is determined. The determination is carried out in closed vessels into which sediment from the wastewater of a factory sump is introduced. In one series of vessels injected surfactant, the other series remains without surfactant. Measure the amount of gas emitted depending on time. The difference recorded in these two series allows us to estimate the biodegradation of surfactants. For most surfactants, an induction period of biodegradation was found, followed by a steep rise in the gas evolution curve, after which the dependence reaches a plateau. A typical test result and criteria that must be met are shown in Fig. 14.

Fig. 14. Criteria for passing the test for biodegradability and typical kinetic curve of biodegradation of surfactants.
Note the long induction period before decomposition begins.
Bioaccumulation
Hydrophobic organic compounds accumulate in the environment, since all biodegradation processes require a specific water environment. Bioaccumulation can be measured directly on fish, but more often it is calculated from model experiments. To do this, measure the distribution of the component between the two liquid phases - octanol and water - and use the logarithmic value lgP. A surfactant is considered bioaccumulative at 1 ^ 0 kt / water\u003e 3. Most surfactants are characterized by lgP values.< 3, поэтому биоаккумулирование не рассматривается как опасная экологическая проблема.
The values of lgP are known and collected for many surfactants; they can be used to estimate the hydrophilicity of surfactants: the lower the lgP value, the higher the hydrophilicity of the surfactant. The hydrophilicity of surfactants is useful to consider when composing compositions. Often, for the same purpose, they use a more well-known standard based on the concept of hydrophilic-lipophilic balance. HLB is widely used for the selection of emulsifiers. There is an inverse proportionality between lgP and HLB: the higher the HLB value, the lower the lgP value. The concept of the critical packaging parameter provides another way to assess the hydrophilicity of surfactants.
Surfactant Marking
According to the recommendations of the Organization for Economic Cooperation and Development, the labeling of surfactants should include aquatic toxicity and biodegradability. Fig. 15 illustrates this procedure. Properties based on biodegradability and toxicity in water
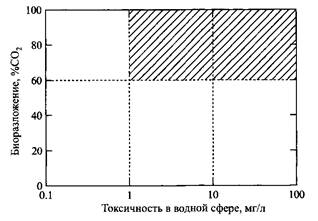
Fig. 15. Ecological classification of surfactants
environment. Shaded areas of the diagram correspond to the permissible values of environmental indicators
most of the currently commonly used surfactants lie in the border area. Hence, the desire of researchers to replace them with components whose properties would allow substances to be located on this diagram “higher and to the right” is understandable.
Tests for aquatic toxicity, biodegradability and bioaccumulation undoubtedly give a complete picture of the effect of surfactants on the environment. There are other parameters that are useful to determine for assessing the environmental impact of surfactants. In addition, the final product often contains a mixture of surfactants or a mixture of surfactants and polymer. Since it is well known that the physicochemical behavior of surfactants in such mixtures is very different from the behavior in an individual solution, it can be expected that the biological effects will be different. For a complete picture, environmental tests must be carried out for all final products.
Table 8. Additional important factors that are useful to consider, along with toxicity in the aquatic environment, biodegradability and bioaccumulation for assessing the ecotoxicity of surfactants
The rate of biodegradation and the structure of surfactant molecules
Consider some parameters that affect the rate of biodegradation of surfactants. First of all, it is necessary that the surfactant be sufficiently soluble in water. Too lipophilic surfactants, such as fluorinated derivatives, accumulate in the lipid tissues of the body and are very slowly destroyed. As mentioned above, many surfactants are sufficiently soluble in water, so the bioaccumulation of the original surfactant does not pose a big threat. However, the initial stage of biodegradation can end with the formation of intermediate products, sparingly soluble in water. A well-known example is the class of ethoxylated alkylphenols, which are destroyed due to oxidative cleavage from the hydroxyl end of the polyoxyethylene chain. In this case, ethoxylated alkyl kilols are formed with polar groups of only a few oxyethylene fragments. Such compounds are very lipophilic and decompose extremely slowly. The study of fish in contact with ethoxylated nonylphenols showed a high level of accumulation of nonylphenols with two and three oxyethylene groups in lipid tissues. This data was one of the reasons for strict control over the influence of surfactants of this class on the environment. Fatty alcohol ethoxylates, apparently, are destroyed by various mechanisms, so lipophilic metabolites are not formed in appreciable quantities.
Along with solubility in water, it is necessary that the surfactant molecule contained bonds that are easily destroyed in the processes of enzymatic catalysis. Most chemical bonds are completely destroyed in nature, but it is important that the rate of destruction is high enough to prevent the surfactant and its metabolites from entering unacceptable amounts into the environment. To increase the rate of biodegradation, it has become generally accepted practice to introduce weak bonds into the structure of surfactant molecules. Such easily broken bonds can, in principle, be placed randomly in the surfactant molecule, but for convenience of synthesis, they are usually introduced between the hydrophobic "tail" and the polar group. Typical examples of such bonds are ester and amide bonds, the destruction of which is catalyzed by esterases / lipases and peptidases / acylases, respectively. One might think that ether bonds in non-ionic surfactants are a source of environmental problems, since enzymes that break down ether bonds are not very common in nature. However, it is not. Under aerobic conditions, hydroperoxides are formed in the a-position with respect to the ester bond, and the destruction of molecules occurs with the formation of aldehydes and acids.
The third factor that must be considered in addition to the solubility in water and the presence of destructible bonds is the branching of the non-polar part of the surfactant molecule. The strong branching of hydrocarbon "tails" often leads to a decrease in the rate of biodegradation. This is probably due to the steric hindrances created by the side groups in order for the surfactant molecule to reach the active center of the enzyme. But the picture is not quite clear. Some types of branching appear to be more dangerous than others, which is probably related to the specific features of a particular enzyme. Metal side groups create less difficulty than longer side alkyl chains. But if a surfactant molecule contains many metal branches in a row, as in poly derivatives, such compounds become dangerous from an environmental point of view. A convincing proof of the value of the linearity of the alkyl chains is the difference in the rates of biodegradation of alkyl benzene sulfonates with linear and branched chains. As already mentioned, branched alkyl benzene sulfonates, tetra-1,2-propylene derivatives as the alkyl chain, have been used before as the main components in household detergent compositions. They are cheap, effective as surfactants and chemically stable, but too stable if viewed from an environmental point of view. When in the 1960-1970s environmental problems arose, these surfactants were quickly replaced by analogs with linear alkyl chains. Linear alkyl benzene sulfonates are satisfactorily degraded under aerobic conditions. However, the speed of their anaerobic biodegradation is relatively low, so researchers are making efforts to overcome this difficulty. It was established that the ability of surfactants to biodegrade strongly depends on the position of branching in the hydrocarbon chain. Branching off of carbon atoms that are two atoms apart from the cleavable bond is less detrimental to biodegradation than branching from the carbon atom separated by a single atom from the bond to be broken. All of the above is important because oxo alcohols, which are widely used as raw materials for the synthesis of surfactants, contain a large amount of 2-alkyl-branches. It has been established that the length of the 2-alkyl side chain has virtually no effect on the rate of biodegradation. Nevertheless, a lot of work will still be required to learn how to predict the biodegradation of surfactants, based on the chemical formula of its molecules.
Environmental protection as an incentive to search for new safe surfactants
All major types of surfactants exist around us for decades. The production methods are optimized, and the physicochemical properties are relatively well studied. Along with the constant task of finding ways to reduce the cost of production of existing surfactants, the development of surfactant chemistry in recent years has been influenced by a market with increasing demand for green products. Today we can note two distinct trends in research aimed at the synthesis of new surfactants: 1) the synthesis of surfactants from natural building blocks, 2) the synthesis of surfactants with collapsing bonds. Below we briefly consider how the production of surfactants from natural building blocks is developing.
Polar groups
Two types of natural products have been investigated for use as surfactant polar groups - carbohydrates and amino acids. Surfactants can be obtained using organic and enzymatic synthesis, or a combination of both methods of synthesis. The greatest efforts were aimed at obtaining surfactants with polar groups from carbohydrate.
In the past few years, research has focused on three classes of surfactants, in which the polar groups are carbohydrate or polyhydric alcohol - alkyl polyglucosides, alkyl glucamides, and sugar esters. The structures of the molecules of these three types of surfactants are presented in Fig. sixteen.

Fig. 16. Structure of some typical surfactants derived from polyhydric alcohols
Currently, there is great interest in the study of alkyl glucosides in connection with the prospects for their widespread use. Such surfactants are synthesized by a direct reaction of the interaction of glucose with a fatty alcohol using a large excess of alcohol to minimize the oligomerization of the carbohydrate. An alternative method is the transacetalization of short-chain alkylglucosides when interacting with long-chain alcohols. In both processes, acid is used as a catalyst. The feedstock is glucose or fractions of hydrolyzed starch. Fig. 17 illustrates such a synthesis. Alkyl glucosides can be obtained by enzymatic synthesis with P-glucosidase; as a result of the reaction, only the P-anomer is obtained in small yields. The corresponding a-anomer can be easily obtained by hydrolysis of the racemate catalyzed by p-glucosidase. a, p-Blend, which is a product of organic synthesis, is significantly different from the pure enantiomers, resulting biocatalytic way. The p-anomer n-octylglucoside has been used as a surfactant in biochemical studies.
Alkyl glucosides are stable at high pH and sensitive to low pH, at which they hydrolyze to carbohydrate and fatty alcohol. The carbohydrate part of the surfactant molecule is more soluble in water and less soluble in hydrocarbons than the corresponding polyoxyethylene blocks; thus, alkylpolyglucosides and other surfactants derived from polyhydric alcohols are more lipophobic than polyoxyethylene surfactants. This makes the physicochemical behavior of alkyl glucoside surfactants in oil-water systems quite different compared to the behavior of ordinary non-ionic surfactants. Moreover, alkyl glucoside surfactants do not exhibit a pronounced inverse temperature dependence of solubility characteristic of non-ionic surfactants. And this leads to large differences in the properties of the solutions of the two types of surfactants under consideration. The main attraction of alkyl glucoside surfactants lies in their favorable ecological properties: they are characterized by high speeds

Fig. 17. Methods of producing alkyl glucose surfactants
biodegradation and non-toxic to water bodies. In addition, these substances, being mild surfactants, do not have undesirable effects on the skin and eyes, which makes this class of surfactants very attractive for personal care products, although they have found a wide range of other technological applications.
Alkiglucamides are commercially important products. The product consumed in huge quantities in detergents is N-dodecano-il-K-methylglucamine, i.e., C12 derivative. It is produced from glucose, methylamine, hydrogen and methyl laurate in two stages. Physico-chemical properties and other characteristics of surfactants of this class are similar to those for alkyl glucosides. However, while alkyl glucosides are extremely resistant to alkalis and labile to the action of acids, alkyl glucamides are also resistant to alkalis, but also relatively resistant to acids.
Glucose esters can be obtained either by enzymatic synthesis using lipase as a catalyst or by organic synthesis. With an optimal enzyme selection, the bio-organic pathway can lead to esterification almost exclusively at the 6-position of the carbohydrate residue. Organic synthesis requires the use of protecting groups to provide the necessary selectivity. Selective enzymatic synthesis of esters of other carbohydrates without prior protection of groups occurs with difficulty. At the same time, proceeding from acetals of carbohydrates and fatty acids, it is possible to obtain monoesters of carbohydrates with a good yield, and after the stage of deprotection from a part of the starting materials it is possible to obtain mono- and di-derivatives. All carbohydrate esters are very labile in an alkaline environment and very stable in acidic environments. Decay products are natural products. Thus, carbohydrate esters are an ideal candidate as a food surfactant, these substances seem to undergo rapid biodegradation, despite the size of the carbohydrate group and regardless of the length of the acyl chain. Sulfonated carbohydrate esters have also been obtained. It turned out that such anionic surfactants undergo biodegradation at lower rates.
Surfactants based on polyatomic alcohols have many attractive properties: they are soft to the skin, non-toxic to water bodies, their biodegradation proceeds at high speeds, it is easy to work with them, they are tolerant to high concentrations of electrolyte. Some of the characteristic properties of surfactants based on polyhydric alcohols are shown in table 9.
Hydrocarbon Radicals
Fatty acids have been used primarily and for a long time as natural sources of the hydrophobic parts of surfactants, for example, to obtain ethoxylated fatty acids and sorbitan esters of fatty acids. Recently, fatty acids have been used to produce ethoxylated monoethanolamide derivatives.
Table 9. Characteristics of surfactants based on polyhydric alcohols
| 1. | Aerobic and anaerobic biodegradation occur quickly. |
| 2. | Low toxicity in aquatic environment. |
| 3. | Hydroxyl groups are highly lipophobic. In the same time |
| Surfactants with sufficiently long hydrocarbon tails have large | |
| hydrophobicity. By virtue of this, such surfactants exhibit a pronounced | |
| this tendency to be localized at the water-oil interface. | |
| 4. | The effect of temperature on the behavior of solutions is insignificant and contrasts |
| falsely effect of temperature on the ethoxylated surfactants. In drawing up | |
| surfactant mixtures from polyhydric alcohol derivatives and | |
| ethoxylated surfactants are obtained nonionic compositions | |
| surfactants whose phase behavior does not depend on temperature |
which in turn is obtained by aminolysis of fatty acid methyl esters with ethanolamine. Ethoxylated fatty acid amides are of interest as an alternative to fatty alcohol ethoxylates for the following reasons: 1) they readily biodegrade to form fatty acids and poly with aminated ends, 2) amide bond in surfactant molecule promotes packing of surfactant due to hydrogen bond formation, 3) double bonds in chains of fatty acids chains are stored in the product.
It is known that double bonds in hydrocarbon tails increase CCM due to an increase in the hydrophilicity of surfactants, as well as due to an increase in the chain volume, which makes packing in dense aggregates and the formation of hydrogen bonds between amide groups difficult. The presence of one double bond has little effect on the cross-sectional area of molecules on the surface. Two double bonds significantly increase this area. Ethoxylates of amides of fatty acids with double bonds in the hydrocarbon tail are of interest as polymerizable surfactants.
Sterols are another class of natural substances of interest as a source of hydrophobic blocks for the production of surfactants. A characteristic feature of sterols-based surfactants is the presence of a large hydrophobic group of natural origin, which, due to the almost flat structure of four cycles, contributes to dense packing in the surface layers. Phytosterols are called sterols of plant origin, currently they are already widely used as raw materials for the production of surfactants. Their structure is similar to that of cholesterol, which is an example of sterols of animal origin. Sterols contain a secondary hydroxyl group that can be ethoxylated. However, the alcohol group is sterically protected, and the reaction with ethylene oxide does not directly proceed. The optimal procedure is as follows: first carry out ethoxylation with a Lewis acid as a catalyst, then stop the reaction after adding 3-5 moles of ethylene oxide and continue the reaction again, but using KOH as the initiator.
Ethoxylated sterols are large, hard molecules, so a long time is needed to form an equilibrium surface layer, and it takes several hours to achieve an extremely low surface tension. It is also possible that the long equilibrium time is associated with the exchange of sterol ethoxylates with different polyoxyethylene chain lengths at the water-air interface and slow conformational changes of molecules on the surface. The structure of the sterols is rigid, and the time required to achieve optimal conformation in the surface layer can be very long. Sterine-based surfactants are of interest as solubilizers and emulsifiers in the manufacture of drugs and cosmetics.
Surfactants are compounds that influence the amount of surface tension. In the process of interaction of liquid molecules between them are formed adhesion forces. This force will be different in the surface and internal (deep) layers. Considering the state of the liquid, it is easy to establish that the particles that are directed into the system are surrounded from different sides by the same molecules that affect them. The resultant of all the forces that act on such a molecule is zero. Therefore, liquids have the smallest surface at a given volume. This is clearly manifested in the spherical shape of the droplets. The presence of impurities of various compounds in liquids causes the magnitude of the surface tension.
The structure of surfactant molecules
Particles of fatty acids and alcohols consist of two parts, which have different properties, so these compounds are often called diphilic structures. One part of the molecule is represented by a hydrocarbon chain, and the other is represented by different functional groups (amino, hydroxyl, carboxyl, sulfo). The longer the hydrocarbon chain, the stronger the particles will be expressed, they will interact less with water.
Surfactants of organic origin: proteins, soaps, alcohols, ketones, aldehydes, tannids, ketones, etc. Surface-inactive substances do not affect surface tension (starch, glucose, fructose).
Nonionic surfactants (nonionic surfactants) are high-molecular biocompounds that do not form ions in water. These substances enter water bodies together with industrial (chemical, textile, household use (use of various household products), as well as waste from agricultural land (herbicides, fungicides, insecticides, and folios as emulsifiers).
Surfactants: harm and benefits
Surface tension is of paramount importance for the absorption processes in the intestine. For example, fats and lipids in the alimentary canal come in the form of drops. The latter are emulsified in the small intestine with the help of bile acids. Only after this, these fats are hydrolyzed by lipolytic enzymes. Very often, soap (surfactants) is added to increase the effectiveness of insecticides. The manipulation allows insecticides to interact better with the surface of the body of insects. However, surfactants have not only positive but also negative effects on the body. For example, the shampoo contains very harmful blowing agents (surfactants), such as sodium and ammonium lauryl sulfate, ammonium and sodium laureth sulfate. It is believed that these components have a carcinogenic effect.
Surfactants have a polar (asymmetric) structure of molecules that can adsorb at the interface of two media and lower the free surface energy of the system. Completely minor surfactant additives can change the surface properties of the particles and give the material new qualities. The effect of surfactant is based on the phenomenon of adsorption, which leads simultaneously to one or two opposite effects: reduction of interaction between particles and stabilization of the interface between them due to the formation of an interfacial layer. Most surfactants are characterized by a linear structure of molecules, the length of which significantly exceeds the transverse dimensions (Fig. 15). Molecule radicals consist of groups related in their properties to solvent molecules, and from functional groups with properties that are sharply different from them. These are polar hydrophilic groups, having pronounced valence bonds and having a definite impact on wetting, lubricating and other actions related to the concept of surface activity . This decreases the stock of free energy with the release of heat as a result of adsorption. The hydrophilic groups at the ends of hydrocarbon non-polar chains can be hydroxyl - OH, carboxyl - COOH, amino - NH 2, sulfo - SO and other strongly interacting groups. Functional groups are hydrophobic hydrocarbon radicals characterized by side valence bonds. Hydrophobic interactions exist independently of intermolecular forces, being an additional factor contributing to the convergence, “sticking” of non-polar groups or molecules. The adsorption monomolecular layer of surfactant molecules with the free ends of hydrocarbon chains is oriented from
the surface of the particles and makes it non-wettable, hydrophobic.
The effectiveness of the action of a surfactant additive depends on the physicochemical properties of the material. A surfactant that produces an effect in one chemical system may not have any effect or, obviously, the opposite effect in another. It is very important the concentration of surfactants, which determines the degree of saturation of the adsorption layer. Sometimes, high-molecular compounds exhibit a similar effect to surfactants, although they do not alter the surface tension of water, such as polyvinyl alcohol, cellulose derivatives, starch, and even biopolymers (protein compounds). The effect of surfactant may have electrolytes and substances that are insoluble in water. Therefore, to define the concept of "surfactant" is very difficult. In a broad sense, this concept refers to any substance that in small quantities significantly changes the surface properties of a dispersed system.
The classification of surfactants is very diverse and in some cases contradictory. There have been several attempts at classification according to various criteria. According to Rebinder, all surfactants according to the mechanism of action are divided into four groups:
- wetting, defoamers and blowing agents, i.e., active at the liquid-gas interface. They can reduce the surface tension of water from 0.07 to 0.03–0.05 J / m 2;
- dispersants, peptizers;
- stabilizers, adsorption plasticizers and thinners (viscosity reducers);
- detergent substances with all the properties of surfactants.
Abroad, the classification of surfactants according to their functional purpose is widely used: thinners, wetting agents, dispersants, deflocculants, frothers and defoamers, emulsifiers, stabilizers of disperse systems. Binders, plasticizers and lubricants are also distinguished.
According to the chemical structure, surfactants are classified depending on the nature of hydrophilic groups and hydrophobic radicals. The radicals are divided into two groups - ionic and non-ionic, the first can be anionic and cationic.
Nonionic surfactants contain non-ionizing end groups with high affinity for the dispersion medium (water), which usually include oxygen, nitrogen, and sulfur atoms. Anionic surfactants - compounds in which a long hydrocarbon chain of molecules with low affinity for a dispersion medium is part of the anion formed in an aqueous solution. For example, COOH is a carboxyl group, SO 3 H is a sulfo group, OSO 3 H is an ether group, H 2 SO 4, etc. The anionic surfactants include carboxylic acid salts, alkyl sulfates, alkyl sulfonates, etc. Cationic substances form in aqueous solutions cations containing a long hydrocarbon radical. For example, 1-, 2-, 3-, and 4- substituted ammonium, and others. Examples of such substances can be salts of amines, ammonium bases, etc. Sometimes a third group of surfactants is distinguished, which includes amphoteric electrolytes and ampholytic substances, which, depending on from the nature of the dispersed phase can exhibit both acidic and basic properties. Ampholytes are insoluble in water, but are active in non-aqueous media, such as oleic acid in hydrocarbons.
Japanese researchers propose a classification of surfactants according to their physicochemical properties: molecular weight, molecular structure, chemical activity, etc. The gel-like shells on solid particles arising from surfactants as a result of different orientations of the polar and non-polar groups can cause various effects: dilution; stabilization; dispersion; defoaming; binding, plasticizing and lubricating actions.
Positive effect of surfactant has only a certain concentration. On the issue of the optimal amount of surfactant administered, there are very diverse opinions. P. A. Rehbinder indicates that for particles
1–10 µm the required amount of surfactant should be 0.1–0.5%. In other sources, values of 0.05–1% or more are given for different dispersity. For ferrites, it was found that for the formation of a monomolecular layer with a dry grinding of surfactants, it is necessary to take a rate of 0.25 mg per 1 m 2 of the specific surface of the initial product; for wet grinding - 0.15–0.20 mg / m 2. Practice shows that the concentration of surfactant in each case should be chosen experimentally.
The technology of ceramic SEM can distinguish four areas of use of surfactants, which allow to intensify the physico-chemical changes and transformations in materials and to manage them in the process of synthesis:
- intensification of the processes of fine grinding of powders to increase the dispersion of the material and reduce the time of grinding when the specified dispersion is achieved;
- regulation of the properties of physicochemical dispersed systems (suspensions, slips, pastes) in technological processes. Here, the processes of liquefaction (or a decrease in viscosity with an increase in fluidity without a decrease in moisture content), stabilization of rheological characteristics, defoaming in disperse systems, etc, are important;
- management of the process of flare formation during spraying of suspensions upon receipt of specified sizes, shape and dispersion of the spray pattern;
- an increase in the plasticity of the molding masses, especially those obtained when exposed to elevated temperatures, and the density of the manufactured blanks as a result of the introduction of a complex of binders, plasticizers and lubricants.
Surface-active substances (surfactants) are such chemicals that are able to concentrate on the phase boundaries and reduce the surface (interfacial) tension. Surfactants are used in pharmaceuticals and cosmetics, in the manufacture of shampoos and foam detergents.
Chemical structure of surfactants
The surfactant molecule consists of a hydrophobic hydrocarbon radical and a hydrophilic polar (functional) group, i.e. the molecule is diphilic, as a result of which it has a high adsorption capacity. For example, in a water / oil emulsion at the interface, the hydrophilic group of the surfactant molecule is oriented toward water, and the hydrocarbon portion toward oil. And the interfacial tension at the same time decreases, which provides stabilization of oil droplets in water.
The detergent effect of surfactants is based on the fact that the surface-active ingredients of lotions, shampoos, soaps are adsorbed on the surface of such contaminants as grease and solid particles, envelop and facilitate their transfer to the washing solution. Surfactants facilitate the spreading of water on the skin surface or products based on them by reducing the interfacial tension.
Types of surfactants
The classification of surfactants is based on the division depending on the nature of the polar group: non-ionic, not dissociating in water into ions, and ionic, which, depending on the charge formed during dissociation of ion in water, are subdivided into: anionic, cationic, amphoteric.
Anionic surfactants, when dissolved in water, form negatively charged ions with a long hydrocarbon chain (organic anions) and a common cation. Anionic surfactant emulsifiers are very effective:
- when creating oil / water emulsions;
- when dispersing a number of powdered materials;
- when used in foaming agents to ensure high foaming in hard water.
An example of anionic surfactant, which is often used in cosmetic formulations, in particular detergents, is sodium lauryl ethoxide (according to INCI nomenclature “Sodium Laureth Sulfate”). It is produced by sulphating saturated or unsaturated primary higher alcohols, followed by neutralization with sodium alkali, ammonia or triethanolamine. Often made in the form of a pasty mass containing up to 70% of the basic substance.
When dissolved in water, cationic surfactants form positively charged ions (organic cations) and a low molecular weight anion. Cationic surfactants include salts of fatty amines and quaternary ammonium bases. Cationic emulsifiers, compared with anionic, less effective because they reduce the surface tension to a lesser extent. But they show bactericidal activity, interacting with the cellular proteins of bacteria. Cationic surfactants are widely used in hair care products (shampoos, conditioners, conditioners for hair). Aliphatic cationic surfactants with one and two hydrocarbon tails are good antistatic agents and are used in cosmetics for hair.
Amphoteric surfactants, depending on the pH of the medium, behave in an alkaline medium as anion-active or in an acidic medium as cation-active. In their molecules, there are functional groups that can have both negative and positive charge. Such surfactants are well compatible with cationic and anionic. Amphoteric surfactants act dermatologically on the skin, which is why they are often used in children's shampoos without tears and for sensitive skin. For example, in combination with anionic surfactant sodium lauryl sulfate, its dermatological rigidity is almost completely softened. Amphoteric surfactants have good foaming.
Betains are one of the varieties of amphoteric surfactants. They belong to the soft and highly foamed surfactants. Amphoteric surfactant cocamidopropyl betaine (Dechiton / Betadet) is included in cosmetics in the production of shampoos, gels and cream-gels, liquid soap, cleansing bath foams. This surfactant contributes to the compatibility of cosmetics with the skin, while improving the viscosity and foaming of this tool. Thus, Dehiton, especially in children's washing-up products, is an emollient component and contributes to the safety of using detergent.
Non-ionic (non-ionic) surfactants are surfactants that, when dissolved in water, do not form ions. They, in comparison with anionic ones, having a weaker foaming ability, have a milder effect on the skin. Such surfactants are often used as emulsifiers, dispersants, solubilizers, and also as co-surfactants, foam stabilizers, wetting agents, etc. An example of a non-ionic surfactant is fatty acid diethanolamides. Used in the manufacture of shampoos and foaming agents in an amount up to 3% as a perezhiruyuschey additive, foam stabilizer and thickener.
In Russian-made shampoos, various combinations of surfactants are used depending on the purpose of the cosmetic product to achieve the required consumer properties and improve the quality.
Surfactants used in the cosmetic industry must comply with the Unified sanitary-epidemiological and hygienic requirements for goods subject to sanitary-epidemiological supervision (control).
The advantages of using surfactants:
- lead to the stabilization of the dispersed system, make it impossible for the sticking and coagulation of the particles of the dispersed phase;
- facilitate the dispersion process and the preparation of cosmetic compositions;
- improve the wettability and flowability of cosmetic substances on the skin;
- ensure the stability of inverse emulsions;
- in the composition of the washing means they improve their foaming and increase the stability of the foam during use.
Literature
Surfactants and compositions. Directory. Edited by M.Yu. Pletnev 2002. - pp.40-44.
Basics of cosmetic chemistry. Basic positions and modern ingredients. Ed. Puchkova T.V. 2011, p.122-133.
Explanatory Dictionary of Cosmetics and Perfumery v.1 Finished products 2nd ed. 2004. p.20.

