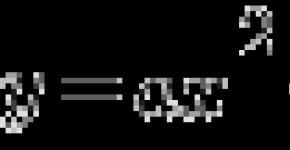What is a manganese: we study the chemical element. Degree of oxidation
Chemistry metals
Lecture 2. Basic issues considered in lectures
Metals VIIIB subgroups
The overall characteristics of metals VIIB-subgroup.
Chemistry manganese
Natural connections MN.
Physical I. chemical properties Metal.
MN connections. Redox properties
Brief characteristic TC and RE.
Executor: | Event number | ||||||||||||||||

Me Talla VIIB-subgroup
general characteristics
VIIIB -Proups form D-elements: MN, TC, RE, BH. |
|||||||||||
Valence electrons are described by the general formula: |
|||||||||||
(N-1) D 5 NS2 | |||||||||||
Simple substances - metals, silver-gray |
|||||||||||
manganese | |||||||||||
severe, with high melting temperatures, which |
|||||||||||
rise during the transition from Mn to RE, so that by tag |
|||||||||||
flochiness Re is inferior only W. |
|||||||||||
MN has the greatest practical significance. |
|||||||||||
technetium | TC, BH elements - radioactive elements, art |
||||||||||
commonly obtained as a result of nuclear synthesis; Re - |
|||||||||||
rare element. | |||||||||||
TC and RE elements are more similar to each other than |
|||||||||||
with manganese. At TC and RE is more resistant to the highest |
|||||||||||
oxidation stump, so these elements are distributed |
|||||||||||
strange compounds to oxidation degree 7. |
|||||||||||
For Mn are characterized by oxidation degrees: 2, 3, 4, |
|||||||||||
More stable - | 2 and 4. These oxidation degrees |
||||||||||
manifest in natural connections. The most common
strange MN minerals: MnO2 pyrolymp and comes Mnco3.
Connections Mn (+7) and (+6) - strong oxidizers.
The greatest similarity of Mn, Tc, Re is shown in high degree oxides
it is expressed in the acidic nature of higher oxides and hydroxides.
Executor: | Event number | ||||||||||||||||
Higher hydroxides of all elements of VIIIB subgroups are strong
acid with general formula NEO4.
In the highest oxidation of the elements Mn, Tc, Re are similar to the similarity with the element of the main subgroup of chlorine. Acids: HMNO4, HTCO4, HREO4 and
HCLO4 are strong. For elements of the VIIIB subgroup characteristic of
similarities with their neighbors for a number, in particular, Mn shows similarities with Fe. In nature, the MN connections are always adjacent to FE connections.
M AR Ganets
Characteristic oxidation degrees
Mn valence electrons - 3D5 4S2. |
|||
The most common degrees |
|||
3D5 4S2. | manganese | oxidation in Mn are 2, 3, 4, 6, 7; |
|
more resistant - 2 and 4. IN aqueous solutions |
|||
the degree of oxidation is +2 resistant to acidic, and +4 - in |
|||
neutral, weakly alkaline and weakness.
Connections Mn (+7) and (+6) exhibit strong oxidative properties.
The acid-primary nature of oxides and hydroxides Mn is natural
varies depending on the degree of oxidation: into the degree of oxidation +2 oxide and hydroxide are the main, and in the highest oxidation - acid,
moreover, HMNO4 is severe acid.
In aqueous solutions, Mn (+2) exists in the form of aquatication
2+, which for simplicity denote Mn2 +. The manganese in high degrees of oxidation is in the solution in the form of tetraoxoanions: MNO4 2- and
MnO4 -.
Executor: | Event number | ||||||||||||||||

Natural compounds and metal
Element Mn for prevalence in the earth's crust among heavy metal
locks follows the hardware, but is noticeably inferior to him, the FE content is about 5%, and Mn is only about 0.1%. Manganese has more common oxide
and carbonate and ores. Minerals are the greatest importance: pyro
whit MnO2 and comes Mnco3.
to receive MN.
In addition to these minerals, Gusmanit MN3 O4 is used to obtain MN
and hydrated ppno2 ppno2 oxide. XH2 O. In manganese ores
Manganese is mainly used in the production of special varieties of steels with high strength and stroke resistance. Therefore,
the main amount of Mn is not in pure form, but in the form of ferromargangan
cA - alloy of manganese and iron containing from 70 to 88% Mn.
The total volume of the annual world production of manganese, including in the form of ferromarganz, ~ (10 12) million tons / year.
To obtain a ferromargangz oxide manganese ore recovered
carbon carbon.
MnO2 + 2C \u003d Mn + 2Co
Executor: | Event number | ||||||||||||||||

Together with Mn oxides, the oxides of the FE are restored, contained in
de. To obtain a manganese with a minimum content of Fe and C, connections
Fe pre-separated and get mixed oxide Mn3 O4
(MNO. Mn2 O3). It is then restored by aluminum (pyrolyzit reacts with
Al too rapidly).
3mn3 O4 + 8Al \u003d 9mn + 4Al2 O3
Pure manganese is obtained by a hydrometallurgical way. After the preload of salt MNSO4, through a solution of Mn sulfate
lust electricity, Manganese is restored at the cathode:
Mn2 + + 2e- \u003d Mn0.
Simple substance
Manganese - light gray metal. Density - 7.4 g / cm3. Melting point - 1245O S.
It is pretty active metal, e (Mn | / Mn) \u003d - 1.18 V. |
||
It is easily oxidized to the Mn2 + cation in the diluted |
|||
acids. | |||
Mn + 2H + \u003d Mn2 + + H2 |
|||
Manganese is passivated in concentrated |
|||
nitric and sulfuric acids, but when heated |
|||
Fig. Manganese - | begins to interact slowly with them, but |
||
ry metal similar | even under the action of such strong oxidants |
||
on iron |
|||
Mn goes into the cation |
|||
Mn2 +. When heated, the powdered manganese interacts with water with
highlighting H2.
Due to oxidation on air, the manganese is covered with brown spots,
In the oxygen atmosphere, the manganese forms oxide |
||||||||||||||||||
Mn2 O3, and at a higher temperature mixed MNO oxide. Mn2 O3. |
||||||||||||||||||
(Mn3 O4). | ||||||||||||||||||
Executor: | Event number | |||||||||||||||||

When heated, the manganese reacts with halogens and gray. Affinity MN.
it is greater than that of iron, so when adding ferromarganese to steel,
the sulfur dissolved in it is associated with MNS. MNS sulfide does not dissolve in metal and goes into the slag. Steel strength After removing the sulfur causeing fragility, increases.
With very high temperatures (\u003e 1200 ° C) Manganese, interacting with nitrogen and carbon, forms nonstociometric nitrides and carbides.
Compound manganese
Manganese compounds (+7)
All connections Mn (+7) exhibit strong oxidative properties.
Permanganate potassium KMNO.4 - the most common compound
mn (+7). In pure form it crystalline substance dark-
purple color. When heating the crystalline permanganate, it is broken
2kmnO4 \u003d K2 MnO4 + MnO2 + O2 |
||
In this reaction in the laboratory you can get |
||
Anion MNO4 - staining solutions permanent |
||
ganat in raspberry purple color. On |
||
tops in contact with mortar |
||
Fig. Solution kmno4 rose | KMNO4, due to the ability of permanganate oxides |
|
purple | tasty water, thin yellow-brown are formed |
|
mnO2 oxide films. |
||
4kmnO4 + 2H2 O \u003d 4mnO2 + 3O2 + 4KOH |
||
To slow down this reaction accelerating in the light, KMNO4 solutions
nat in dark bottles.
When adding several drops of concentrate to crystals permanganate
the tried sulfuric acid is formed by manganese anhydride.
Executor: | Event number | ||||||||||||||||
2kmnO4 + H2 SO4 2mn2 O7 + K2 SO4 + H2 O
Oxide Mn 2 O 7 is a heavy oily liquid of dark green. This is the only metal oxide, which conventional conditions naho-
g. liquid state (Melting point 5.9 0 s). Oxide has a mole
the chular structure, very unstable, at 55 0 s decomposes with an explosion. 2mn2 o7 \u003d 4mnO2 + 3O2
Oxide Mn2 O7 is a very strong and energetic oxidizer. Many
ganic substances are oxidized under its exposure to CO2 and H2 O. Oxide
Mn2 O7 is sometimes called chemical matches. If a glass wand moisten in Mn2 O7 and bring to the alcohol, it will light up.
When Mn2 O7 is dissolved in water, manganese acid is formed.
Acid HMNO 4 is a strong acid, exists only in water
mind solution is not highlighted in free state. HMNO4 acid decomposes
with the release of O2 and MnO2.
When adding a solid alkali to the KMNO4 solution occurs
green manganate.
4kmnO4 + 4KOH (K) \u003d 4K2 MnO4 + O2 + 2H2 O.
When heated KmnO4 with a concentrated hydrochloric acid form
gas CL2.
2kmnO4 (K) + 16HCl (conc.) \u003d 2mnCl2 + 5Cl2 + 8H2 O + 2KCL
In these reactions, strong oxidative properties of permanganate are manifested.
KMNO4 interaction products with reducing agents depend on the acidity of the solution in which the reaction proceeds.
In acid solutions, a colorless Mn2 + cation is formed.
MnO4 - + 8H + + 5e- Mn2 + + 4H2 O; (E0 \u003d +1.53 V).
From neutral solutions, a brown sediment MnO2 falls.
MnO4 - + 2H2 O + 3E- MnO2 + 4OH-.
In alkaline solutions, the green Anion MnO4 2 is formed.
Executor: | Event number | ||||||||||||||||

Permanganate potassium in industry is obtained either from manganese
(oxidizing it on an anode in an alkaline solution), or from pyrojitis (MNO2
it is oxidized to K2 MnO4, which then oxidized to KMNO4 on the anode).
Manganese compounds (+6)
Manganats - Salts with Anion MnO4 2-, have bright green.
Anion MNO4 2─ Sustainable only in strong alkaline environment. Under the action of water and, especially, manganate acids are disproportionated to form
mn into the degree of oxidation 4 and 7.
3mnO4 2- + 2H2 O \u003d MnO2 + 2mnO4 - + 4OH-
For this reason, the acid H2 MnO4 does not exist.
Manganats can be obtained by fusion of MNO2 with alkalis or carbonate
mi in the presence of the oxidant.
2mnO2 (K) + 4KOH (g) + o2 \u003d 2K2 MNO4 + 2H2 O
Manganats are strong oxidizing agents but if they are
to give an even stronger oxidant, they go to permanganates.
Disproportionation
Manganese compounds (+4)
- The most stable MN connection. This oxide is found in nature (mineral pyrojitis).
MnO2 oxide - black and brown matter with very durable crystalline
hively grid (the same as Rutile TiO2). For this reason, despite the fact that MNO 2 is amphoteric, It does not react with alkalis solutions and with diluted acids (as well as TiO2). It dissolves in concentrated acids.
MnO2 + 4HCl (conc.) \u003d MnCl2 + CL2 + 2H2 O
The reaction is used in the laboratory to obtain CL2.
When dissolving MnO2 in concentrated sulfur and nitric acid MN2 + and O2 are formed.
Thus, in a very acidic environment, MnO2 seeks to go to
mn2 + cation.
MnO2 alkalis reacts only in melts to form mixed
oxides. In the presence of an oxidant in alkaline melts, manganats are formed.
MnO2 oxide is used in industry as a cheap oxidizing agent. In particular, oxidative and restorative interaction
Executor: | 2 decomposes with O2 release and |
Executor: | Event number | ||||||||||||||||
PART 1
1. The degree of oxidation (p. O.) Is The conditional charge of the atoms of the chemical element in the complex substance calculated on the basis of the assumption that it consists of simple ions.
You should know!
1) in connections with. about. hydrogen \u003d +1, except hydrides .
2) in connections with. about. oxygen \u003d -2, except peroxides  and fluorides 
3) The degree of metal oxidation is always positive.
For metals of the main subgroups of the first three groups with. about. Constant:
metals IA Group - with. about. \u003d +1,
metals IIA group - with. about. \u003d +2,
metals IIIA group - with. about. \u003d +3. four
At free atoms and simple substances from. about. \u003d 0.5.
Total with. about. All elements in the compound \u003d 0.
2. Method for the formation of titles Two-element (binary) compounds.

4. Complete the table "Names and formulas of binary connections".

5. Determine the degree of oxidation of the highlighted element of the complex compound.

PART 2
1. Determine the degrees of oxidation of chemical elements in compounds according to their formulas. Write down the names of these substances.

2. Divide the substances FEO, Fe2O3, CaCl2, ALB3, CUO, K2O, BACL2, SO3 into two groups. Record the names of substances by indicating the degree of oxidation.

3. Set the correspondence between the title and the degree of oxidation of the atom of the chemical element and the compound formula.

4. Make the formula of substances by name.

5. How many molecules are contained in 48 g of sulfur oxide (IV)?

6. With the help of the Internet and other sources of information, prepare a message on the use of any binary connection according to the following plan:
1) formula;
2) name;
3) properties;
4) Application.
H2O water, hydrogen oxide. Water under normal conditions liquid, without color, odor, in a thick layer - blue. Boiling temperature is about 100 ° C. It is a good solvent. It consists of a water molecule of two hydrogen atoms and one oxygen atom, it is its high-quality and quantitative composition. This complex substance is characterized by the following chemical properties: interaction with alkaline metals, alkaline earth metals.
Water exchange reactions are called hydrolysis. These reactions are of great importance in chemistry.
7. The degree of oxidation of manganese in the compound K2MNO4 is equal to:
8. The smallest degree of oxidation chromium has in the compound, the formula of which:
1) SG2O3
9. The maximum degree of oxidation chlorine exhibits in the compound, the formula of which:
Olympic tasks for chemistry
(1 School Stage)
1. Test
1. The only degree of oxidation, the manganese has in connection
2. Neutralization reactions correspond to the abbreviated ion equation
1) H + + OH - \u003d H 2 O
2) 2H + + CO 3 2- \u003d H 2 O + CO 2
3) Cao + 2H + \u003d Ca 2+ + H 2 O
4) Zn + 2H + \u003d Zn 2+ + H 2
3. Interact with each other
2) MNO and Na 2
3) P 2 O 5 and SO 3
4. The equation of the redox reaction is
1) KO + HNO 3 \u003d KNO 3 + H 2
2) N 2 O 5 + H 2 O \u003d 2 NNO 3
3) 2N 2 O \u003d 2N 2 + O 2
4) Vaso 3 \u003d Vao + CO 2
5. The exchange reaction is interaction
1) calcium oxide with nitric acid
2) carbon monoxide gas
3) ethylene with oxygen
4) hydrochloric acid with magnesium
6. Acid rains are caused by the presence in the atmosphere
1) nitrogen and sulfur oxides
4) Natural Gas
7. Methane, along with gasoline and diesel fuel, is used as a fuel in internal combustion engines (vehicles). The thermochemical burning equation of gaseous methane has the form:
CH 4 + 2O 2 \u003d CO 2 + 2N 2 O + 880 KJ
What amount of the heat of heat is highlighted during CH 4 combustion, the volume of 112 liters (with N.U.)?
Choose the correct answer:
2. Tasks
1. In the equation of the redox reaction, lay the coefficients by any method known to you.
SNSO 4 + KMNO 4 + H 2 SO 4 \u003d Sn (SO 4) 2 + MNSO 4 + K 2 SO 4 + H 2 O
Specify the names of the oxidant and reducing agent substance and the degree of oxidation of the elements. (4 points)
2. Write the reaction equations that allow the following transformations:
(2) (3) (4) (5)
CO 2 → CA (HCO 3) 2 → Caco 3 → CAO → CaCl 2 → Caco 3
(5 points)
3. Determine the formula of the alkadien, if its relative air density is 1,862 (3 points)
4. In 1928, the American chemist of General Motors Research (General Motors Research), Tomas Midgle, was able to synthesize and allocate a chemical compound in its laboratory, which consisted by 23.53% of carbon, 1.96% hydrogen and 74,51 % fluorine. The resulting gas was 3.52 times heavier than air and did not burn. Output the compound formula, write the structural formulas of organic substances corresponding to the resulting molecular formula, give them the name. (6 points).
5. Mixed 140 g of 0.5% hydrochloric acid solution with a 200 g of a 3% solution of hydrochloric acid. What is the percentage of hydrochloric acid in the newly obtained solution? (3 points)
3. Crossword
Sold out words encrypted in crossword
Designations: 1 → - horizontally
1 ↓ - vertically
↓ Iron corrosion product.
→ formed when interacting (6) with basic oxide.
→ Unit of the amount of warmth.
→ Positively charged ion.
→ Italian scientist, whose name is named one of the most important permanent values.
→ Number of electrons on external level Element number 14.
→ ...... gas - carbon oxide (IV).
→ The Grand Russian scientist is famous, including both the creator of mosaic cloth, the author of the epigraph.
→ Type of reaction between sodium hydroxide solutions and sulfuric acid.
Give an example of the reaction equation for (1 →).
Specify the constant value mentioned in (4).
Write the reaction equation (8).
Write electronic structure atom elementwhich is mentioned in (5). (13 points)
Electronic configuration of an unexcited manganese atom - 3D 5 4S 2; The excited state is expressed by the electronic formula 3D 5 4S 1 4P 1.
For manganese, the compounds are most characteristic of the oxidation degree +2, +4, +6, +7.
Manganese - silver-white, fragile, quite active metal: in a row of stresses it is between aluminum and zinc. On the air, the manganese is covered oxide film, preventing it from further oxidation. In a small-barred state, the manganese is easily oxidized.
MNO manganese oxide (II) and the corresponding hydroxide Mn (OH) 2 has basic properties - with acids with acids, bivalent manganese salts are formed: Mn (OH) 2 + 2 H + ® Mn 2+ + 2 H 2 O.
Mn 2+ cations are also formed when dissolving metal manganese in acids. Manganese (II) compounds show reducing properties, for example, a white precipitate Mn (OH) 2 in air quickly darkens, gradually oxidizing to MNO 2: 2 Mn (OH) 2 + O 2 ® 2 MNO 2 + 2 H 2 O.
Manganese oxide (IV) MNO 2 is the most stable compound of manganese; It is easily formed both when oxidizing manganese compounds at a lower oxidation (+2) and when restoring manganese compounds in higher oxidation degrees (+6, +7):
Mn (OH) 2 + H 2 O 2 ® MNO 2 + 2 H 2 O;
2 KMNO 4 + 3 Na 2 SO 3 + H 2 O ® 2 MnO 2 ¯ + 3 Na 2 SO 4 + 2 KOH.
MNO 2 - amphoteric oxide, however, and acidic, and its basic properties are weakly expressed. One of the reasons for the fact that MNO 2 does not show clearly expressed basic properties, is its strong oxidative activity in an acidic medium (\u003d +1.23 V): MnO 2 is restored to Mn 2+ ions, and does not form resistant salts of quadricular manganese. The appropriate manganese oxide (IV) hydrate should be considered as hydrated manganese dioxide MNO 2 × XH 2 O. Manganese oxide (IV) as an amphoteric oxide formally correspond to the ortho-and meta-form not selected in the free state of manganese acid: H 4 MNO 4 - Orto-shape and H 2 MNO 3 - meta-form. The manganese oxide Mn 3 O 4 is known, which can be considered as a salt of a bivalent manganese of the ortho-shape of a manganese-sicted acid Mn 2 MNO 4 - manganese orthomaganite (II). In the literature there are reports on the existence of Mn 2 O 3 oxide. The existence of this oxide can be explained by considering it as a salt of a bivalent manganese meta-shape of a manganese acid: MNMNO 3 - manganese methanganite (II).
When weaving in an alkaline medium of manganese dioxide with such oxidizing agents, both chlorate or potassium nitrate occurs the oxidation of a four-collar manganese to a hexavalent state, and a potassium manganate is formed - salt is very unstable even in a solution of manganese acid H 2 MNO 4, which (MNO 3) is unknown:
MNO 2 + KNO 3 + 2 KOH ® K 2 MNO 4 + KNO 2 + H 2 O.
Manganats are unstable and prone to disproportionation by reversible reaction: 3 K 2 MNO 4 + 2 H 2 O ⇆ 2 KMNO 4 + MNO 2 ¯ + 4 Koh,
as a result, the green color of the solution due to manganate ions MNO 4 2- changes to the purple color characteristic of MNO 4 permanganate ions.
The most widely used compound of seven-willed manganese is potassium permanganate KMNO 4 - salt known only in HMNO 4 manganese solution. Potassium permanganate can be obtained by the oxidation of manganats with strong oxidizing agents, for example, chlorine:
2 K 2 MNO 4 + Cl 2 ® 2 KMNO 4 + 2 KCl.
Manganese oxide (VII), or manganese anhydride, Mn 2 O 7 - explosive green-brown liquid. Mn 2 O 7 can be obtained by reaction:
2 kmno 4 + 2 H 2 SO 4 (conc.) ® Mn 2 O 7 + 2 kHSO 4 + H 2 O.
The compounds of manganese in the highest oxidation is +7, in particular permanganates, are strong oxidizing agents. The depth of recovery of permanganate ions and their oxidative activity depends on the pH of the medium.
In the sylnic acid medium, the permanganate recovery product is the Mn 2+ ion, and the bivalent manganese salts are obtained:
MnO 4 - + 8 H + + 5 E - ® Mn 2+ + 4 H 2 O (\u003d +1.51 V).
In a neutral, weakly alkaline or weakness medium, MNO 2 is formed as a result of the recovery of permanganate ions:
MnO 4 - + 2 H 2 O + 3 E - ® MNO 2 ¯ + 4 OH - (\u003d +0.60 V).
MNO 4 - + 4 H + + 3 E - ® MNO 2 ¯ + 2 H 2 O (\u003d +1,69 V).
In a strongly algae, permanganate ions are restored to MNO 4 2 manganate ions, while the type K 2 MNO 4, Na 2 MNO 4 is formed:
MNO 4 - + E - ® MNO 4 2- (\u003d +0.56 V).