Phosphate powders are a health hazard. What are surfactants surfactants
Toggle navigation
Professional chemistry for home and business

It's no secret for anyone that the majority of detergents consist of surface-active substances (surfactants). What is it, how they are classified and what difference do we try to figure out.
Surfactants - surfactants, they are also tensides, they are surfactants - organic compounds with amphiphilic structure, i.e. surfactant molecules contain both hydrophilic and hydrophobic components, due to which they have the property of reducing the surface tension of water. It is this quality that determines the specifics of the use of surfactants as the main component of most detergents. Simplified the effect of surfactants can be described as follows. With its hydrophobic end, the surfactant molecule “catches” pollution molecules (if, of course, they have hydrophobic nature - alkanes, fats, oils and the like), reducing the mutual attraction forces between pollution molecules, at the same time the hydrophilic end of the surfactant molecule reaches for water. Thus, emulsified impurities are washed away from the surface.
By type of hydrophilic groups, surfactants are divided into 4 types:
Anionic - in aqueous solution, they decompose to form negatively charged ions. They have excellent detergent properties, form abundant foam and reduce surface tension well, but they are destroyed in an acidic environment, sensitive to water hardness, and have a high irritant effect. Traditionally used as the basic components of virtually any detergent, both household and industrial use. The production of anionic surfactants exceeds the production of all other surfactants combined. The biodegradability of anionic surfactants is quite high (80-95%), however, due to the high foaming capacity, it is necessary to control their disposal, so there is a limit on the content of anionic surfactants in the water of reservoirs to 0.5 mg / l.
Cationic - in an aqueous solution, they decompose to form positively charged ions. The most toxic among all surfactants. Possess low washing ability. The use of cationic surfactants is due to their adsorption on virtually any surface - anti-corrosion components, antistatic agents, anti-caking agents, conditioners, bactericides, dispersants and softeners are the main uses.
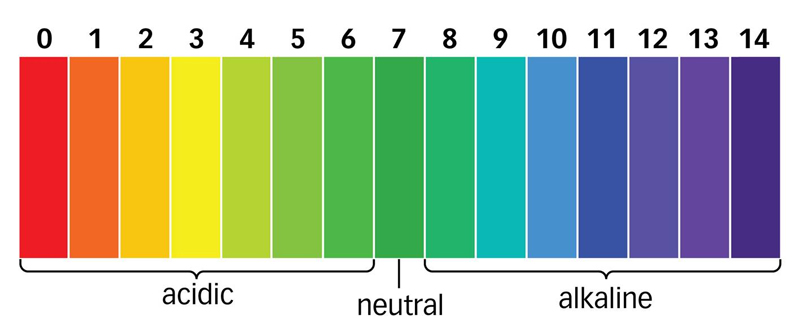
Amphoteric - in aqueous solution, depending on the pH of the medium, can be cationic (in an acidic environment, pH<7) или анионные (в щелочной среде рН>7) properties .. Amphoteric surfactants have a high foaming, mild dermatological effect on the skin and are widely used in shampoos “without tears”, in washing means for children and for people with sensitive skin. In household and professional chemistry, this type of surfactant is valued for its excellent rinsing properties and the absence of stains on the surface.
Non-ionic - in aqueous solution do not form ions. The least toxic surfactant. Possess greater biodegradability (up to 100%) and a weak irritant effect compared with other types of surfactants. Non-ionic surfactants are insensitive to water hardness, have high surface activity, have excellent detergent properties, and form little foam.
In addition, there is a classification of surfactants according to the nature of use (detergents, emulsifiers, wetting agents, solubizers), by the length of the hydrophilic chain (hydrophobic and hydrophilic), and also by origin (natural and synthetic).
The most interesting is the last classification, since the recent trend of environmental friendliness promotes "Green chemistry from 100% natural raw materials." Many manufacturers seeking to improve their rating and compliance with the latest trends, actively use the Eco trend in their own interests.
The term “natural surfactant” refers to a natural source of a substance. Nevertheless, despite assurances from manufacturers of household and professional chemicals, not a single surfactant produced now in substantial amounts can be considered natural in the full sense. With a few exceptions, all tensides are produced in the process of organic synthesis, and often in very harsh conditions, when by-products are inevitably formed. For example, monoglycerides are widely distributed in nature, but surfactants, marketed as monoglycerides, are obtained during industrial hydrolysis of triglyceride oils at temperatures above 200 ° C, which leads to the formation of glycerol di-and tri-derivatives. For example, alkyl glucoside is one of the most common and widely used surfactants in detergents. Household chemistry manufacturers appreciate high foaming and good emulsifying ability, as well as the opportunity to write on the label: “BIO”, “ECO” and “From natural renewable raw materials” (especially gifted PR people do not skimp on the allegory and on some means you can find inscriptions: "Made from sugar cane" or even like this: "Natural surfactants from straw and bran"). However, we should not forget that these surfactants, however, are obtained using multi-stage chemical processes, affecting 100% natural and renewable raw materials with various (and not always harmless) reagents.
In order to properly assess the origin of surfactants, it is useful to divide them into two classes depending on the raw materials from which they are derived: oleochemical and petrochemical surfactants. Oleochemical surfactants are produced from vegetable oils. Petrochemical surfactants are produced from small "building blocks", such as ethylene, obtained by cracking oil. Often the raw material for surfactants at the same time are vegetable oils and petrochemicals. Ethoxylated fatty acids are one of numerous examples.
The production of surfactants using vegetable oils as a raw material does not always ensure the production of less toxic and less environmentally harmful surfactants than petrochemical productions. Taking into account the carbon cycle, chemical production based on renewable raw materials is always more preferable. However, overpaying for eco-labeling and advertising tricks of manufacturers, in our opinion, is not worth it ...
Reviews
And I continue to get acquainted with the line of tools of the Russian manufacturer under the TM Dirtoff. Recently, a new tool has arrived in our shop under the house - a universal one. To be honest, I doubted for a long time whether to buy it or not, since the price is rather “biting”, and the application is, well, very vague. I read on the manufacturer's website - it is written that the windows should be washed, and
Dirtoff Universal and matt kitchen))I somehow always biased towards universal means, considering that it is impossible to create one tool that would wash all surfaces. But it so happened that I had to visit the Interbythim exhibition and get acquainted with the Russian manufacturer of household and professional chemicals - Dirtoff (a friend decided to open a cleaning company and we went to look at the new products in the field of chemistry). Managers so
Chic means Dirtoff UniversalNatalia Makarenko: “I received the Dirtoff Textile for carpet and textile cleaning for testing. Volume - 750 ml, quite large, given the economical consumption due to the spray. Manufacturer: LLC “Elite Agrosystems”, Russia, Moscow region, the city of Voskresensk. Applied this tool on two types of materials. My first test was on the carpet. Before using on the carpet manufacturer recommends vacuuming. Need to
“TEST-DRIVE” Concentrated cleaner for carpets and textiles “Dirtoff Textile” (Russia), 750 mlFeedback on the Dirtoff Grill. For the first time I tried Dirtoff Grill. I used it on the dirtiest part of the kitchen - a wardrobe near the stove, which was all spattered with grease and there was no hope of washing it. I decided to try a new domestic product and was pleasantly surprised. I applied the remedy (from the trigger) and lightened it for 10 minutes. During this
Catherine Ashastova, a young motherFeedback on the Dirtoff Textile tool. Our poor couch moved with us three times already and even fell under the rain during one of the shipments. Given its light color, as well as the properties of the fabric with which it is upholstered, this piece of furniture in general can be called quite capricious. Let us add the conditions in which he lives, and we get a constantly dirty sofa. At this stains
Anna, blogger
Household chemicals - the undoubted achievement of civilization. Without it, it is difficult to do. However, maintaining cleanliness, we underestimate the harm that chemistry can do to our health. Most of the synthetic detergents used contain hazardous substances that cause skin irritation and inflammation of the mucous membranes of the eyes and nose, difficulty breathing, coughing and asthma attacks, increase the risk of allergies and even cancers.
Before buying detergents, be sure to pay attention to their composition indicated on the package and carefully read the instructions. Do not use household chemicals that contain chlorine, organochlorine compounds, phosphates and phosphonates - these substances are dangerous to the environment and often to human health.
The range of some household chemicals that do not contain chlorine and phosphates can be found in the manual "". We will be grateful if you help us complete this list. Information can be sent to an address marked “Green Office”, and it may be included in future editions of this manual.
There are other criteria for the safety of household chemicals. The same manual contains a list of companies producing and / or selling household chemicals that meet some of these criteria, for example, the content of natural components, biodegradability.
The safest products are those that maximally decompose in the environment into safe components. Use of safe household chemicals Chemical safety Chlorine and its organic compounds can cause diseases of the cardiovascular system, contribute to the occurrence of atherosclerosis, anemia, hypertension, and can adversely affect the condition of the skin and hair. They also increase the risk of allergies and, in some cases, the risk of cancer. In the EU, most chlorine-containing compounds have been banned since 1987.
Phosphates and phosphonates are found in most detergents as a water softener and can cause allergic skin reactions and damage to the respiratory tract. In addition, phosphates, entering natural reservoirs, serve as fertilizer for algae and cause flowering, which leads to the death of most of their inhabitants. Currently, many manufacturers refuse to introduce phosphate additives into household cleaning products, replacing them with more environmentally friendly substances - zeolites and polycarboxylates.
The names of most chemical terms speak little to the uninitiated, so we will show you.
In addition to chlorine, phosphates and phosphonates, the following are dangerous:
Anionic Surfactants (detergents and laundry detergents). Surface-active substances (surfactants) clean dishes and surfaces from dirt (detergents), and are also used in laundry detergents. Surfactants are of three main types: anionic, cationic and non-ionic. The most dangerous - anionic (A-surfactant). They cause immunity disorders, allergies, damage to the brain, liver, kidneys, and lungs. Keep in mind, when using detergents, surfactant gets into your body, as even a ten-time rinse in hot water does not completely free the dishes from chemicals. To reduce the harmful effects, use products in which the surfactant content does not exceed 5%.
Sodium hypochlorite - sodium hypochlorite (bleach). Chlorine is very dangerous, and this chemical compound is very unstable and easily “releases” chlorine. It is the cause of diseases of the cardiovascular system, contributes to atherosclerosis, anemia, hypertension, allergic reactions, adversely affects the skin and hair, increases the risk of cancer.
Oil Distillates (in polishes for metal surfaces): short-term exposure may cause temporary visual impairment; long-term exposure leads to impaired functioning of the nervous system, kidneys, organs of vision and skin diseases.
Ammonia (cleaning agents for glass surfaces): irritates eyes, respiratory tract, causes headaches.
Phenols and cresols (bactericides) are very corrosive, cause diarrhea, dizziness, loss of consciousness and impaired kidney and liver function.
Nitrobenzene (in polishes for floors and furniture): causes skin discoloration, shortness of breath, vomiting, and in severe cases - death; exposure to this substance causes cancer, it is the cause of birth defects in children.
Formaldehyde (preservative in various products): is a carcinogen; Irritant to eyes, throat, skin, respiratory tract and lungs.
In addition, try to avoid chemical products with the following icons:
Annoying
Typically, this icon can be found on jars with various cleaning products and detergents. After contact with eyes and skin, this product can cause itching, irritation and even inflammation. Avoid contact with eyes and wash hands thoroughly after handling this substance. Also try to work in well ventilated areas - evaporation of these products can cause coughing and inflammation of the airways.

Caustic
For example, various means for cleaning sewer pipes may be caustic. A warning sign indicates that a large amount of alkali or acid is present in the product. This means that if it comes in contact with the skin, it can cause severe burns and serious damage to the skin, muscle tissue and mucous membrane. When working with such products it is necessary to wear gloves.
If possible, use safe alternatives to chemicals.
After all, to achieve perfect purity and brilliance is quite possible and natural means, without resorting to the help of chemistry. For example:
A plunger can handle a clogged bathroom no worse than special chemicals.
Baking soda - natural remedy with which the dishes can be brought to shine. Soda will help you effectively deal with stains, clean and polish aluminum, chrome, silver, steel, tin and plastic surfaces, as well as jewelry. It can be used to clean and deodorize refrigerators, badly soiled and foul-smelling carpets, upholstery on furniture and vinyl. Soda also softens tissues and removes some types of stains. Baking soda softens hard water, so you can take a relaxing bath with it. Soda can be used as a deodorant for the body and as a toothpaste, it can be used as a means of scaling.
Lemon juice can be used to remove rust stains from dishes, as well as polishing table silver. Lemon can be used when washing glass and removing stains from aluminum, clothing and porcelain. Lemon juice can bleach when exposed to sunlight.
Lovers of pleasant smells should go with air fresheners for natural natural oils and essences (lemon, eucalyptus, spruce, orange, lavender). In order to freshen the air in the room, you can use special aromatic lamps, which include a maximum of 20 minutes, dropping there in advance only a few drops of aromatic essence.
Vinegar effectively removes wax stains and stains from all sorts of resins, perfectly disinfects (pure vinegar can be used to treat the toilet), cleans tiles and tiles (just do not forget to ventilate the room), removes scum (pour a little vinegar into the kettle with water, stir, rinse - and you're done!). In addition, an efficient and safe “glass cleaner” can be prepared from vinegar, which cleans perfectly and leaves no stains. To do this, just dilute two teaspoons of vinegar in 1 liter of water. Vinegar can also be used to clean bricks and stones.
Borax. It is a natural mineral, soluble in water. Borax prevents the formation of powdery mildew and mold, improves the cleaning properties of soap and other cleaning agents, removes stains, and if it is mixed with sugar or something sweet, you can fight with cockroaches.
Myzen It is made from corn and can be used for cleaning windows, polishing furniture, cleaning carpets and starching clothes.
What really washes ?!
Surface active substances (surfactants) are, as a rule, chemicals that are contained in any cleaning agent, even in regular soap. Just thanks to the surfactant cleaning agent cleans. What are surfactants for?
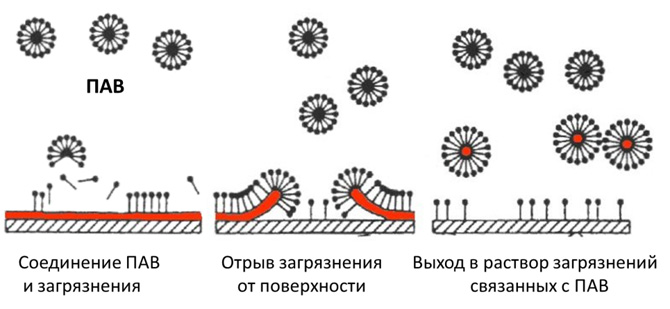
Dirt, especially grease, is very difficult to wash off with water. Water will drain without washing off the fat. Water molecules do not stick to fat molecules and do not take them with them. Therefore, the task is to attach fat molecules to water molecules. This is exactly what surfactants do. The surfactant molecule is a sphere, one pole of which is lipophilic (it combines with fats), and the other is hydrophilic (it comes into contact with water molecules). That is, one end of the surfactant particle is attached to the fat particle, and the other end to water particles.
How do surfactants affect our skin?
However, most of the moisture in the human body also has a fatty basis. Those. for example, the protective layer of the skin (lipids - fats that protect the skin from various bacteria in the body) is a fatty film and is naturally destroyed by surfactants. And the infection attacks the place that is least protected, which of course is harmful to human health. Surfactants also destroy the body's cells (the activity of destruction depends on the type of surfactant). Experts say that after applying the detergent, the protective layer of the skin should have time to recover within 4 hours to at least 60%. These are the standards of hygiene established by state standard specification. However, not all detergents provide such a recoverable skin. Fat-free and dehydrated skin ages faster. In addition, surfactants can accumulate in the brain, liver, heart, body fat (especially a lot) and continue the destruction of the body for a long time. And since practically no one does without detergents, surfactants are constantly replenished in our body, providing continuous harm to the body. Surfactants also affect reproductive function in men, similar to radioactive radiation. The problem is aggravated by the fact that our wastewater treatment plants do not cope well with the removal of surfactants. Therefore, harmful surfactants are returned through the water supply system to us almost in the same concentration in which we pour them into the drain. The only exceptions are products with biodegradable surfactants.
Types of surfactants, features and which of them are biodegradable
According to the chemical nature of the hydrophilic group (soluble in water), surfactants are divided into four large classes: Anionic surfactants - in aqueous solution, they decompose to form negatively charged ions. The main advantage is the relatively low cost, efficiency and good solubility. But they are the most aggressive towards the human body. Cationic surfactants - in aqueous solution, decompose to form positively charged ions, possess a bactericidal property. Among cationic surfactants, quaternary ammonium compounds (QACs), imidazalines, fatty amines have the greatest value. Non-ionic surfactants - do not form ions in aqueous solution. The main advantage is a favorable effect on the fabric and most importantly - 100% biodegradability. Amphoteric surfactants - in aqueous solution, depending on the pH of the medium, can be cationic (in an acidic environment, pH<7) или анионные (в щелочной среде рН>7) properties. Some of the anionic (anionic) surfactants, for example, alkyl sulfonates, possess good biodegradability (by 80-98%). But nonionic surfactants have the most complete (100%) biodegradability. The inclusion of non-ionic surfactants in the formulation of detergents leads to a lower content of anionic substances on the skin. A similar effect, namely, a decrease in the accumulation of anionic surfactants on the skin and tissues, was established with the introduction of enzymes of biological origin in detergent compositions. One of the main criteria for the environmental safety of household and professional chemicals is the biodegradability of surfactants, which are included in their composition. The company "Viyusa Bel" uses exclusively anionic and non-ionic surfactants. There are primary biodegradability, which implies structural changes (transformation) of surfactants by microorganisms, leading to the loss of surface-active properties. By complete biodegradability are meant the final biodegradation of surfactant to carbon dioxide and water.
The main use of surfactants is as an active component of detergents and cleaners (including those used for decontamination), soap, for the care of premises, equipment, tools, dishes, clothing, things, cars, etc. Currently, the most common surfactant in synthetic detergent is an alkyl benzene sulfonate. The group of anionic surfactants also includes alkanesulfonate (SAS), alkyl sulfate (FAS) and volatile alkyl sulfate (FAES). FAS can be obtained from vegetable raw materials, such as rapeseed oil, or coconut oil. In cationic surfactants, the hydrophilic group is represented by a positively charged, nitrogen-containing group. The negatively charged counterweight is chlorine ion, or methyl sulfate.
Why do you need a co-surfactant?
Since the actual detergent action is formed mainly by anionic surfactants, they form the basis of any detergent. The most common in the production of liquid soap is ethoxylated sodium laureth sulfate. A softer, but expensive substance - ethoxylated magnesium laureth sulphate - is usually used in baby cosmetics and products for sensitive skin. Oxyethylated ammonium laureth sulfate is often used in detergents manufactured in the USA. In European products, it is less common, since in these countries its dermatological properties are considered not high enough. Unfortunately, in the domestic market there are still widely distributed products containing only sodium laureth sulfate as a washing substance. These are very tough products, and whatever useful additives (most often plant extracts) are included in their recipe, they do not correct the situation. For the best removal of dirt, dissolving excess sebum, restoring the protective lipid layer with dermatological softness and good foaming, you must use a mixture of surfactants. Therefore, in addition to the main surfactant, Co-surfactant is always added to the detergent formulation. This is what helps to create an optimal balanced composition. As Co-surfactants, various amphoteric, non-ionic, anionic, and cryptoanionic surfactants are used. The ratio of surfactant and Co-surfactant is usually 1: 3, 1: 4. In practice, developers have to work out such ratios, taking into account the required cosmetic and technical characteristics. Sometimes with a combination of absolutely safe surfactants, the mixture exhibits undesirable dermatological effects. High demands on the purity of the environment induce the creation of biodegradable surfactants (biosurfactants). The fact is that synthetic detergents are practically not subject to natural decomposition processes, therefore they are able to accumulate in the environment, causing considerable damage to nature. Biodegradable surfactants are metabolic products of bacteria or components of membranes, that is, substances that in the external environment break down into the original non-toxic constituents. An attempt to create biosurfactants was carried out abroad. They are obtained by biotechnology in the process of growing various microorganisms: bacilli, fungi, pseudomonads. On a chemical structure, they are very diverse, but have a common advantage - they are safe from an environmental point of view.
On the packaging with detergents, there are THERE THREE SECURITY SIGNS: "The sign of the cycle in nature." Safe packaging materials are used. Packaging can be recycled and reused. In addition, in nature, it decomposes without forming hazardous chemical compounds, such as dioxin. Thus, the purity of the soil and water is maintained. “Apple” - “Safety conscious formula”. Used composition is safe for our health and the environment. This mark is placed on the packaging of creams, personal care products, shampoos, etc. The hairsprays on which this sign stands do not use gases such as propane and butane. Varnishes are sprayed by mechanical action, and not by the pressure that propane, butane or other gases create in a hermetically sealed cartridge. When spraying varnish, we do not inhale these gases, and they do not enter the environment. “Bunny” - “No animal testing”. Not tested on animals. This is not necessary, the manufacturer uses only safe components. And thus no harm to animals. Typically, an animal test is needed in order to select the concentration of chemical components that will not lead to dangerous consequences for the animal, and therefore for humans.
"The sign of the cycle in nature." Safe packaging materials are used. Packaging can be recycled and reused. In addition, in nature, it decomposes without forming hazardous chemical compounds, such as dioxin. Thus, the purity of the soil and water is maintained. “Apple” - “Safety conscious formula”. Used composition is safe for our health and the environment. This mark is placed on the packaging of creams, personal care products, shampoos, etc. The hairsprays on which this sign stands do not use gases such as propane and butane. Varnishes are sprayed by mechanical action, and not by the pressure that propane, butane or other gases create in a hermetically sealed cartridge. When spraying varnish, we do not inhale these gases, and they do not enter the environment. “Bunny” - “No animal testing”. Not tested on animals. This is not necessary, the manufacturer uses only safe components. And thus no harm to animals. Typically, an animal test is needed in order to select the concentration of chemical components that will not lead to dangerous consequences for the animal, and therefore for humans.
The most commonly used surfactants for skin cleansing are the main active ingredients of detergents, and the strength of the agent’s effect (mild or irritant) depends on them. Basically, when creating detergents, anionic (negatively charged) surfactants are used as surfactants, as they have the best ability to foam and soap. Since the detergents in the form of briquettes are hard because of the need to preserve the shape and structure, which should not change during the production process, it is possible to use only certain surfactants. In the manufacture of liquid detergents, on the contrary, there is a larger selection of active chemical components. In addition, the production process of liquid detergents is such that emollients can be included in them in larger volumes than in solid detergents. Structural formulas of surfactants most often used in the creation of solid and liquid detergents.
Surfactants in solid detergents
The main surfactant found in most solid detergents worldwide is soap (alkyl carboxylate). Soaps, also called natural surfactants, are usually formed during the saponification of fats, during which an interaction occurs between the triglyceride and the alkaline compound. In the production of soap, vegetable oils are commonly used, such as palm oil, palm oil derivatives (palm stearin, palm olein), rice, peanut or castor oil in combination with coconut or palm oil. The components of non-plant origin used to create soap are usually made from animal fats, such as rendered lower grade fats. Although soaps are effective as cleansers, they negatively affect the condition of the skin. The use of soap, especially in countries with a cold climate, is associated with the appearance of erythema, xerosis and itchy skin.

The ability of detergents to irritate the skin depends on several factors. The type of surfactant used is critical. Surfactants with a molecular chain length from C8 to C14 are the most active components in solution, so they have the most pronounced irritant effect. The composition of detergents on a soap base usually includes these surfactants. Irritant effect is also due to the fact that these detergents are poorly washed off the skin (while surfactants may remain on the surface) and increase its pH. If the pH of the skin remains elevated for more than 4 hours (for example, when using alkalinity increasing agents, or when washing the skin frequently), the alkalinity index on the surface of the skin increases, which can lead to irritation. The pH value for most soaps ranges from 9.5 to 11.0, which is typical of an alkaline environment. As a result of attempts to reduce the irritant effect of soaping agents by adding additional components to their composition, new types of soaping agents have been developed, such as perezhirayuschie additives, transparent soaps and combined solid soaps.
Soap with perezhirivayuschimi additives.
These soaps are obtained by incomplete saponification (neutralization), during which fatty acids or oils do not react. They can also be synthesized by adding to the soap during the production of fatty alcohols, acids or esters. Usually due to perezhirivayuschih additives improve the properties of soaping means, such as:
softness of action;
the ability to moisturize the skin;
foaming ability;
the expense of means decreases.
Transparent soaps.
The composition of these funds in high concentrations contain moisturizers, such as glycerin. Thanks to these moisturizers that increase the solubility of the soap, it becomes transparent. However, these products are characterized by a high content of active soaping substances and an alkaline pH, which increases their irritant effect. However, transparent soaps are classified as mild soaps, due to the presence of glycerin, which is a moisturizer, and a low content of fatty substances.
Combined solid soaps.

These products usually contain natural soaps and mildly acting synthetic surfactants. Synthetic surfactants reduce the irritant effect of these agents, however, the pH at the same time remains high, approximately at the level of 9.0-9.5. Combined solid soaps are usually less likely to cause skin irritation than standard solid soaps.
Solid synthetic detergents.
Solid soaps consisting of surfactants are called solid synthetic detergents. In contrast to soaping agents, solid synthetic detergents are obtained by esterification, ethoxylation and sulfonation of oils, fats or petroleum derivatives. Solid synthetic detergents typically contain the following synthetic surfactants:
sulfosuccinates;
cocoyl sulfate sodium monoglyceride;
sodium cocoyl isethionate.
alkyl glycerol sulfated esters;
a-olefin sulfonates;
Solid cleansing soaps (containing alkylcarboxylate) have an alkaline pH, the values \u200b\u200bof which range from 10 to 10.5. In contrast, solid synthetic detergents (the main substance alkyl isethionate) have a neutral pH. Additional components of solid synthetic detergents are refractory fatty acids, waxes and esters. It should be noted that sodium cocoyl isethionate, the most commonly used synthetic surfactant, has particular molecular properties that were used in the development of a new direction in the production of detergents, namely, in the creation of mildly active substances.

Surfactants most commonly used in the manufacture of liquid detergents
The composition of liquid detergents usually simultaneously includes anionic and amphoteric (containing a neutral charge) surfactant. Non-ionic surfactants and surfactants, synthesized from amino acids, are more and more often included in the composition of detergents, as they provide a soft action. Anionic surfactants commonly found in liquid detergents include soaps (salts of fatty acids) and synthetic surfactants, such as:
alkyl ether sulfate;
alkylaryl isethionates;
alkyl phosphates;
alkyl sulfosuccinates;
alkyl sulfonates.
In the production of liquid detergents, anionic surfactants derived from amino acids (for example, acylglycinates) are most often used as the main surfactants. The most commonly used zwitterionic surfactants are cocamidopropyl betaine and cocoamphoacetate. One of the non-ionic surfactants that make up some detergents is alkyl polyglycoside. Surfactants derived from amino acids, such as alkyl glutamates, sarcosinates and glycinates, are increasingly used in the manufacture of detergents. Most liquid detergents are characterized by a neutral or acidic pH. The exceptions are the means for creating which soap (alkyl carboxylates) is used as the main active ingredient. The pH of these detergents is alkaline.
Other components included in the composition of the skin wash
In addition to surfactants, detergents contain structuring substances, organoleptic properties modifiers and flavors. Flavoring agents may be the most expensive component of the detergent, but the importance of their presence for consumers cannot be overestimated. In solid detergents, structuring substances are needed to maintain their “solid state” and to facilitate a rather complex production process. Most often, long-chain fatty acids, waxes and alkyl ethers are used as structuring agents. In the production of liquid detergents, structuring substances are used in order to provide the necessary rheological properties / density of the liquid, which affect the flow rate and the particular application of the product. In addition, structuring substances provide physical stability of dispersion-suspension systems and the presence of a moisturizing effect. Softeners are included in the detergent composition in order to achieve a minimum drying effect on the part of the surfactant. Triglyceride oils, fats, liquid paraffin, waxes and mineral oils are used most often in the manufacture of moisturizing shower gels as softening / occlusive agents. To enhance the moisturizing effect, water-soluble moisturizers, such as glycerin, can also be added to the products.

Detergents designed to produce special effects may contain other additional active ingredients. For example, antimicrobial detergents often contain bactericidal agents, such as triclosan or triclocarban. The list of additional active ingredients used to achieve special additional effects is established by the FDA. The FDA controls the safety of detergents and detergents that have an antibacterial effect or other effects similar to those of drugs. The safety of true soaps that do not have other effects is controlled by the Consumer Product Safety Commission. Detergents developed for frequent disinfection of the skin of the hands of health care workers or the food industry should comply with more stringent requirements than the above products. These agents usually contain potent cationic antimicrobial agents, such as chlorhexidine or benzalkonium chloride. Additional components, such as salicylic acid or benzoyl peroxide, are also included in facial cleansers designed to treat acne. Facial cleansers are usually characterized by a relatively low pH. With the development of technologies for the production of detergents, thanks to which it was possible to achieve a positive effect of these funds on the condition of the skin, they began to include additional components, such as nutrients and substances that reduce the severity of the manifestations of aging skin.
Ever wondered how the dishwashing detergent and laundry detergent work and what it consists of?
The problem is that dirt, especially grease, is very difficult to wash off with water. Try to wash greasy hands with water. Water will drain without washing off the fat. Water molecules do not stick to fat molecules and do not take them with them. Therefore, the task is to attach fat molecules to water molecules. This is exactly what surfactants do. The surfactant molecule is a sphere, one pole of which is lipophilic (it combines with fats), and the other is hydrophilic (it comes into contact with water molecules). That is, one end of the surfactant particle is attached to the fat particle, and the other end to water particles.
Surfactant (Surface Active Substances) - These are, as a rule, chemicals that are contained in any detergent, even in ordinary soap. Just thanks to surfactants cleaning agent cleans.
However, most of the moisture in the human body also has a fatty basis. Those. for example, the protective layer of the skin (lipids - fats that protect the skin from various bacteria in the body) is a fatty film and is naturally destroyed by surfactants. And the infection attacks the place that is least protected, which of course is harmful to human health. Surfactants also destroy the cells of the body (the activity of destruction depends on the type of surfactant).
Experts say that after applying the detergent, the protective layer of the skin should have time to recover within 4 hours to at least 60%. These are the standards of hygiene established by state standard specification. However, not all detergents provide such a recoverable skin. Fat-free and dehydrated skin ages faster. In addition, surfactants can accumulate in the brain, liver, heart, body fat (especially a lot) and continue the destruction of the body for a long time. And since practically no one does without detergents, the surfactants are constantly replenished in our body providing continuous harm to the body. Surfactants also affect reproductive function in men, similar to radioactive radiation.
The problem is aggravated by the fact that our wastewater treatment plants do not cope well with the removal of surfactants. therefore harmful surfactants are returned through the water supply to us almost in the same concentration in which we pour them into the drain. The only exceptions are funds with biodegradable surfactants.
Anionic Surfactants - The main advantage is the relatively low cost, efficiency and good solubility. But they are the most aggressive towards the human body.
Cationic Surfactants have a bactericidal property.
Nonionic surfactants - The main advantage is a favorable effect on the fabric and the main thing - 100% biodegradability.
Ampholytic surfactants - depending on the environment (acidity / alkalinity), they manifest themselves either as cationic or as anionic surfactants.
Good biodegradability (80-98%) have some of anionic (anionic) surfactantsfor example, alkyl sulfonates. But nonionic surfactants have the most complete (100%) biodegradability.
The inclusion of non-ionic surfactants in the formulation of detergents leads to a lower content of anionic substances on the skin. A similar effect, namely, a decrease in the accumulation of anionic surfactants on the skin and tissues, was established with the introduction of enzymes of biological origin in detergent compositions.
One of the main criteria for the environmental safety of household chemical goods is biodegradability of surfactantswhich are included in their composition. There are primary biodegradability, which implies structural changes (transformation) of surfactants by microorganisms, leading to the loss of surface-active properties. By complete biodegradability are meant the final biodegradation of surfactant to carbon dioxide and water. But no household chemical goods in our San Epidemiological Stations are tested for biodegradability.
It is believed that no more than 5% surfactant in washing powderso that it is well washed. So read the composition of the powders, the less surfactant, the less harm to health.
In fact, only soap root and saponins (soap substances) from plants can be recognized as a natural detergent.


