Formulas pav. Surface active substances (surfactants)
In the production of ICS, in addition to binders, fillers and fillers, additive substances in mixtures, called additives, are widely used.
At the stages of technological production they are:
- facilitate the implementation of operations;
Reduces the amount of energy expended;
Reduce the consumption of expensive components;
Reduce the consumption of materials;
Contribute to the provision of the necessary indicators of material properties;
Favorable or slowing down the process of structure formation and solidification.
At the stage of operation of the construction of the additive, the ISC introduced earlier are called upon:
Strengthen, stabilize the structure of the material;
Maximum inhibition of the inevitable destruction arising and developing in the material under the influence of the external environment and internal spontaneous phenomena.
The main functional purpose of additives,and by this they differ from fillers and fillers, is thatthat they always actively interact with one or several components of the mixtures in the process of forming the structure of the binding part or macrostructure of the ICS. As a result of the reaction, new compounds appear that were not previously in the mixture, and the additives are either completely consumed or lose their individual traits. It is clear that with an excess amount, the additives may partially remain in the mixture and in the formed material without any changes, which is not desirable.
Surface active substances (Surfactant ) call such chemical compounds that are adsorbed on the interfaces of liquids and solids and affect their physicochemical or chemical properties. Surfactants are, as a rule, compounds whose molecules consist of two main parts - the radical and the functional group.
Radical - represents a group of atoms, which, at a number of chemical transformations, is unchanged and passes from a molecule of one compound to a molecule of another.
Radicals are formed, for example, during the splitting of organic compounds of hydrogen atoms in hydrocarbon molecules. So, if in any limiting (saturated) compound belonging to the class of paraffins of the type C n H 2 n + 2, a hydrogen atom is split off, then the remaining group of atoms C n H 2 n +1 is an aliphatic (fatty) radical
H - C - C - ... - C -, which is denoted by the letter R.
The place of split hydrogen in a molecule can be taken by another atom or group of atoms with certain properties associated with the stationary displacement of electrons in atomic orbits, which causes the presence of a specific electric dipole and dipole moment of the entire molecule. Such atoms or groups of atoms are called functional groups .
The most common functional groups in surfactants are:
Hydroxyl: (- OH);
Carboxyl: (- COOH);
Amine (amino group): (- NH 2);
Nitro group: (- NO 2);
Sulfate group: (- SO 3 H).
By the number of functional groups in the surfactant molecule can be one-, two-and polybasic.
Compounds in which the aliphatic radical contains less than 10 carbon atoms, as a rule, do not possess surface activity, i.e. the ability to adsorb and lower surface tension liquids or surface energy of solids. When the radical contains more than 10 carbon atoms, they are usually surface-active and are called higher fatty surfactants . The solubility of surfactants in various solvents and the ability to dissociate into ions depend on the type of functional polar group and the structure of the radical.
Surfactants in which functional groups carry a positive charge are active in an acidic environment and inactive in an alkaline, whereas surfactants with negatively charged functional groups are, on the contrary, active in an alkaline and inactive in an acidic.
CLASSIFICATION surfactants
In principle, all surfactants are divided into two large groups: inogenic compounds, when dissolved in water, dissociate into ions, and non-inogenic, which do not dissociate into ions.
Depending on which ions cause the surface activity of ionic substances — anions or cations, ionic substances are divided into anion-active, cationic, ampholytic. Anionic surfactants are active in alkaline solutions, cation-active - in acidic, ampholytic - in those and others.
Anion active substances in alkaline solutions, forming negatively charged surface-active ions (anions):
RCOONa ↔ RCOO - + Na +
Cationic substances during dissociation in acidic solutions form positively charged surface-active ions (cations):
RNH 3 Cl ↔ RNH 3 + + Cl -
Anionic surfactants include: carboxylic acids (RCOOH) and their salts (RCOOMe), etc.
Cationic surfactants include amines, ammonium bases:
RNH 2; RNH 3 Cl.
Ampholytic surfactants contain two functional groups, one of which is acidic, the other - the main character, for example, carboxyl and amine groups.
Depending on the medium, ampholytic compounds have anionic or cationic properties:
Alkaline environment is acidic;
RNH (СH 2) n COO - ↔ RNH (СH 2) n COOH ↔ RNH 2 (СH 2) n COOH;
Anionic properties are cationic properties.
Nonionic surfactants, dissolving in water, do not form ions.
The group of noniogenic surfactants includes products of fatty acid ethoxylation, alcohols, amines.
RCOO (C 2 H 4 O) n · H; RCH 2 O (C 2 H 4 O) n · H; RC 6 H 5 O (C 2 H 4 O) n OH.
CLASSIFICATION OF A surfactant by the mechanism of action
Depending on the effect of surfactants in disperse systems, they are divided into 4 groups:
To the first group include low molecular weight, truly water-soluble surfactants, such as alcohols. They are weak wetting and defoamers.
To the second group include surfactant dispersants and emulsifiers. By adsorbing, they effectively lower the free surface energy of a liquid or solid and thereby facilitate the process of formation of new surfaces and dispersion. These substances have some stabilizing effects.
As a result of oriented adsorption, surfactants of the second group hydrophobize solid surfaces and, conversely, hydrophobic surfaces hydrophilize. The effect of the hydrophobization of these surfactants is especially pronounced, which is aggravated by a chemical bond — the fixation of the polar surfactant groups on the corresponding parts of the solid surface.
Second class surfactants include fatty acids, their water soluble salts, cationic organic bases and salts.
In the third group combined surfactants, which are good stabilizers. Their surface activity is relatively small.
These surfactants are also good adsorption plasticizers - plasticize the structure, reducing their strength and structural viscosity. In cement mortars and concretes, this makes it possible to switch to hard and at the same time homogeneous mixtures, promotes uniformity of mixing, increases the density and durability (frost resistance), leads to an increase in strength and a decrease in cement consumption.
Calcium lignosulphonates are used as plasticizers (sulfite-alcohol bard - PRS and sulphite-yeast brew - RRM), etc.
Fourth surfactant group - it is a detergent with high surface activity, wetting and water-repellent action. They are also effective emulsifiers and emulsion stabilizers. This group includes soaps of fatty acids and amines.
In the construction mainly use surface-active substances of the second - fourth groups.
Surfactants for cement concrete mixtures and cement concrete are divided into the following types:
1. Regulating properties of concrete mixes
1.1. Plasticizing 1-4 groups (super-, strongly-, medium- and weakly plasticizing). Increase the mobility of the concrete mix, slow down the setting of concrete and increase strength.
1.2. Stabilizing. Increase the homogeneity of concrete, reduce permeability.
1.3. Water retaining. Increase the mobility of the mixture, reduce the permeability and strength of concrete, increase the uniformity of concrete.
1.4. Improve pumpability. Increase uniformity, reduce water separation of the mixture and the strength of concrete.
1.5. Slow down setting. Increase the time of mobility of the mixture, slow down the setting of 2 or more times at + 20 ° C. Increased strength in the long periods of hardening.
1.6. Accelerating setting. Accelerate the setting by 20% or more at 20 ° C. Acceleration hardening.
1.7. Poizuyuschie - for lightweight concretes.
1.8. Air entraining. Increased workability and frost resistance, reduced stratification.
1.9. Foam and gas generating. Foaming additives provide technical foam. Gas-forming surfactants are capable of emitting gas due to chemical interaction with cement hydration products.
2. Regulating concrete hardening
2.1. Accelerating hardening. Strength increase at the age of 1 day by 20% or more. Slowing down strength at a later date.
2.2 Slow down hardening. Reducing the strength of concrete by 30% or more at the age of 7 days.
3. Increases strength and (or) corrosion resistance, frost resistance of concrete, reduced permeability of concrete
3.1. Water reduction (1-4 groups). Reduced water consumption (by 20-5%). Increase in frost resistance and corrosion resistance.
3.2. Colmatising Improving the brand of concrete for water resistance and corrosion resistance.
3.3. Air entraining and gas generating. Increase in frost resistance in 2 and more times, plasticization of mix.
3.4. Enhancing the protective properties of concrete in relation to reinforcement (steel corrosion inhibitors). Increase in mobility of mix and decrease in diffusion permeability of concrete.
4. Imparting special properties to concrete
4.1. Antifreeze (providing hardening at low temperatures).
4.2. Hydrophobic (1-3 groups). Reduction of water absorption in 1,5-5 times or more, slowing the setting.
The introduction of surfactants in cement paste, mortar or concrete mixture significantly changes their structure and properties in a plastic and hardened state. The various types of surfactants noted above change the properties of a concrete mix or concrete differently due to their adsorption on the surface of clinker grains and new growths, as well as on the surface of stone materials.
The microstructure of the hydrated cement also changes as a result of the developing adsorption modification. The surface of the crystals formed in the cement paste and stone is covered with an adsorption passivating film of surfactants, the growth of crystals is slowed down and a smaller crystalline structure is formed with a change in the very shape of the crystals.
Thus, using surfactants, it is possible to significantly expand the production of asphalt and cement concrete mixtures. In this case, the main thing is the right choice of materials and additives, as well as their dosing.
Figure 1: Surfactants: Scheme of Action
Sodium and potassium salts of higher fatty acids (soaps) are surface-active substances that can form resistant soap films. Surface active substances (surfactants) in a certain way can be located at the interface of two phases, for example, such as water-air or water-oil. Such behavior of surfactants is explained by the peculiarity of their structure: a surfactant molecule, such as soap, includes both a polar, ionizable hydrophilic group, and a non-polar hydrophobic part — the hydrocarbon. At the interface, the hydrophilic group is oriented toward water, and the hydrocarbon radical is toward the oil phase or air.
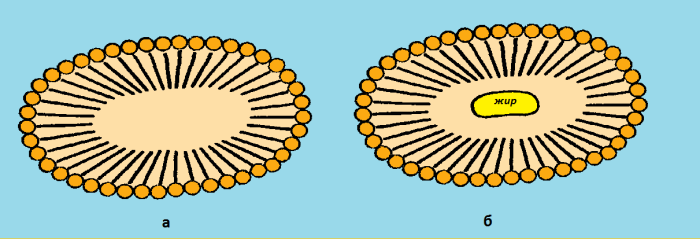
Figure 2: Soap Micelles
In an aqueous medium at a certain concentration, the surfactant molecules no longer exist in the form of isolated particles, but as large aggregates, micelles, in which all hydrocarbons are in the center of the micelles, and hydrophilic groups are outside (Fig. 2a). A micelle is able to “capture” particles of water-insoluble substances and create resistant ones, since the adhesion of micelles is prevented by the same charge of their surfaces (Fig. 2b). The washing action of soaps is based on this principle. Contamination is a fatty film with dust particles. Soaps emulsify impurities, after which the emulsion is easily washed off with water.
Synthetic surfactants and detergents
For the production of soap you need a large amount of fat - a valuable food and technical product. Meanwhile, other organic compounds having a similar structure with soaps have surface activity.
These include:
- anionic surfactant (eg, sodium salts of alkyl sulfates, alkyl sulfonates);
- cationic surfactants (eg quaternary alkylammonium salts)

Figure 3: Formulas for synthetic surfactants
Anionic and cationic surfactants necessarily contain in the molecule a long alkyl radical (C 12 - C 14)
Synthetic surfactants are produced in industry from available hydrocarbons, mainly oil, therefore they are inexpensive. Another virtue detergents on the basis of synthetic surfactants is the possibility of their use in hard water, in which conventional soaps provide insoluble calcium and magnesium salts, which reduces the effectiveness of the washing action and increases the consumption of soap.
Twins

Figure 4: The Twin Formula Formula
Recently, surfactants have found widespread use in industry (for example, in textiles). tweens. In pharmacy, they serve as a synthetic emulsifying base in the manufacture of ointments.
Tweens are built according to a general principle, like other surfactants, that is, there are non-polar and polar parts in their molecules. The basis of the molecule is cyclic tetrahydric alcohol. sorbitanin which from one to three hydroxyl groups are esterified with higher fatty acids. The radicals of these acids make up the non-polar part of the molecule.
The remaining hydroxyl groups form an ether bond with the residues of polyethylene glycol - (CH 2 CH 2 O) n CH 2 CH 2 OHwhere n = 40-80. Fragments of polyethylene glycol represent the polar part of the tweens.
Bibliography: Organic Chemistry, A.P. Luzin, S.E.Zurabyan, N.A. Tyukavkina, 1998
You can buy surfactants (surfactants) we have. Call: (+38 044) 228-08-72.
Surface active substances (surfactants) - chemical compounds, which, concentrating on the interface, cause a decrease in surface tension.
Thanks to detergent, wetting, emulsifying, dispersing and other valuable properties, surfactants are widely used in the production of detergents and cleaning products, cosmetics and pharmaceuticals. Latex. Rubber Polymers. Chemical plant protection products, textiles, leather and paper, building materials, corrosion inhibitors, in the extraction, transportation and processing of oil, etc. Most surfactants (estimated 55-60%) are used for the production of synthetic detergents (SMS).
Currently used synthetic surface-active substances (surfactants) are divided into 4 classes:
- anionic surfactant - compounds that dissociate in aqueous solutions with the formation of anions, causing surface activity. Among them, linear alkyl benzene sulfonate, fatty acid sulphates and sulfoesters are most important;
- amphoteric (ampholytic) surfactants - compounds that are ionized in aqueous solutions and behave depending on the conditions (mainly on the pH-medium), that is, in an acidic solution exhibit the properties of cationic surfactants, and in an alkaline solution - anionic surfactants. Among the main amphoteric surfactants should be noted alkyl betaines, alkylaminocarboxylic acids, derivatives of alkyl imidazolines, alkylaminoalkanesulfonates.
- nonionic surfactants - compounds that dissolve in water without ionizing. The solubility of nonionic surfactants in water is due to the presence of functional groups in them. As a rule, they form nitrates in aqueous solution due to the occurrence of hydrogen bonds between water molecules and oxygen atoms of the polyethylene glycol part of the surfactant molecule. These include: polyglycolic esters of fatty alcohols and acids, polyglycolic esters of fatty acid amides, acylated or alkylated polyglycolic esters of alkylamides.
- cationic surfactants - compounds that dissociate in aqueous solution with the formation of cations that determine surface activity. Among cationic surfactants, quaternary ammonium compounds, imidazalines, fatty amines are most important.
The main raw material for multi-tonnage production of surfactants is oil refining and petrochemical synthesis: low molecular weight and higher paraffins, olefins, synthetic fatty acids, higher fatty alcohols, alkyl derivatives of benzene and phenol, ethylene oxide, etc.
It is well known that the first surfactant - soap - “lives” for almost 4000 years, however in the 50s it gave up its position to alkylbenzene sulfonate-based detergents and cleaning agents. However, 9 million tons of soap is consumed annually in the world. Thus, soap remains the most common surfactant in the world, followed by ABS. Soap, according to strategic marketing estimates, has been in the so-called “saturation phase” for many years. The “phase of degeneration” will probably never come as long as humanity lives.
Surfactant in cosmetics
The concept of “Cosmetics” unites a wide range of various products designed to care for the hair and body of a person. It is a hair shampoo and liquid soap; hair coloring products; means for ear hair after washing; rinses, balms, etc .; cosmetic creams for the face, body, hands, including therapeutic and prophylactic action.
Modern shampoos are multifunctional products that contain various ingredients, providing softness of action, stability, foaming, improving appearance and hair neck.
The basis of the raw components of the shum-punya are surface-active substances (surfactants), as well as various useful additives, including biologically active ones.
Anionic substances are used as the main surfactants, which provide a sufficient washing effect and foaming with a gentle effect on the skin and hair.
For conventional serial shampoos, anionic surfactants (alkyl sulfates and alkyl ether sulfates)
In order to obtain “soft” sham-puny in a mixture with them, alkylamido ether sulfates, sulfosuccinates, and to a lesser extent, isotionates, sarcosinates, etc. are used.
Auxiliary surfactants include amphoteric, non-ionic and cationic substances. They are necessary in shampoo formulations to increase the compatibility of the main surfactants with skin and hair, increase the foaming properties, regulate viscosity, and reduce the depressant effect. For this purpose, imidazoline derivatives, betaines, alkylolamides, oxides of amines are widely used.
Alkylolamides, glycol ethers of fatty alcohols are used as solubilizers for the introduction of dusts and other hydrophobic components (oils, biologically active substances).
Cationic, non-ionic surfactants, beta-yins are used as conditioning agents, removing the charges of static electricity and facilitating combing of dry and damp hair.
The most effective antistatic agents are cationic surfactants — quaternary ammonium compounds, although there are incompatibility problems with anionic surfactants. However, in a mixture with non-ionic and amphoteric substances, we manage to achieve the desired effect and preserve the stability of the finished product.
Amine oxides, alkylphosphate hydroxy esters are also used to soften hair and reduce its electrification.
A separate group of shampoos, liquid soaps, bath foams are particularly “soft” compositions intended for children and adults with sensitive skin, i.e., compositions of increased softness from the point of view of the impact on the skin. Here the requirements for raw materials are particularly high. Most often, a mixture of alkyl ether sulfates with amphoteric surfactants — imidazoline derivatives, as well as betaines and monoalkyl sulfosuccinates — is used as the active principle. The same basis is used in anti-dandruff and therapeutic shampoos.
Anionic Surfactants
The main types of surfactants used in the composition of CMC are alkylbenzene sulfonates with a linear alkyl chain (LABS) and C12-C15 alcohol derivatives (ethoxylates, sulfates, alcohol ethoxy sulfates). LABS and alcohol sulfates, along with soap, belong to anionic surfactants, alcohol ethoxylates - to non-ionic (non-ionic) surfactants.
Nonionic surfactants
The second important type of surfactant for SMS is non-ionic surfactants obtained by oxyethylation of higher fatty alcohols or alkylphenols.
The most commonly used non-ionic surfactants are fatty alcohol oxyethylates, which can be based on both linear and branched alcohol. If ethoxylates based on long chain alcohols (C12-C15), due to better detergency, are more commonly used in CMC laundry recipes, it is preferable to use ethoxylates based on short chain alcohols (C9-C11) to clean solid surfaces. These ethoxylate are distinguished by better wetting ability and wetting angle with respect to hard surfaces. In general, nonionic surfactants due to the variability of their bases and the degree of oxyethylation or propoxylation can ideally be tailored to a specific task. They, as a rule, surpass anionic surfactants both in cleaning and degreasing action and emulsify more or less oils and fats depending on the profile of use.
Amphoteric surfactants
From the group of amphoteric surfactants, betaine derivatives are most often used (for example, cocamopropyl betaine)). In combination with anionic surfactants, they improve the foaming ability and increase the safety of formulations, and when combined with cationic polymers they increase the positive effect of silicones and polymers on hair and skin. These derivatives are derived from natural raw materials, so they are quite expensive components.
We offer such surface active agents (surfactants):
Surfactants (Surfactants)
Surfactants (Surfactants)
- organic compounds whose molecules have a polar hydrophilic part in the structure (functional groups –OH, –COOH, –O, etc.) and a hydrocarbon hydrophobic one. Thus, they are soluble in organic solvents and water.
Micelle
The main quantitative characteristic of surfactants is the ability of a substance to reduce surface tension at the interface. But surfactant has a limit of solubility ("critical micelle concentration" or CMC), with the achievement of which when adding a surfactant to the solution, the concentration at the interface remains constant, but at the same time, self-organization of the surfactant molecules occurs in a bulk solution (or aggregation). As a result of this aggregation, micelles are formed. A distinctive feature of micelle formation is the clouding of the surfactant solution. Aqueous solutions Surfactants, when micelle formation also acquire a bluish tint (gelatinous shade) due to the refraction of light by micelles.
Among anionic surfactants, alkyl aryl sulfonates are the most common. Primary dodecyl sulfate and straight-chain dodecyl benzene sulfonate have optimal surface-active properties. These substances are thermally stable, low-toxic (LD 50 1.5-2 g / kg, white mice), do not irritate human skin and satisfactorily undergo biological degradation in water bodies, with the exception of alkylaryl sulfonates with a branched alkyl chain. They are well combined with others. Surfactants, while showing synergism, their powders are non-hygroscopic. Secondary alkyl sulfates have good foaming ability, but are thermally unstable and are used in liquid form. Secondary alkyl sulfonates have a high surface activity, but are very hygroscopic. Perspective surfactants are those in which the hydrophilic part consists of several functional groups. For example, disodium salts of sulfoic succinic acid have good sanitary and hygienic properties along with high colloid-chemical and technological indicators when dissolved in hard water. Surfactants containing a sulfonylamide group have biological activity. Dodecyl phosphate also has good properties.


