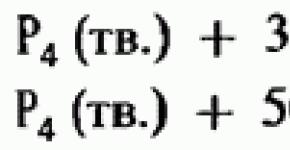Where phosphorus and its compounds are used. Phosphorus compounds
Phosphine is a colorless gas with a characteristic fishy odor. He is very poisonous. To obtain phosphine in laboratory conditions, white phosphorus is boiled in a concentrated aqueous solution of sodium hydroxide:
Phosphine obtained in this way may spontaneously ignite due to the presence of unstable diphosphine in it.
The phosphine molecule has a pyramidal shape similar to the ammonia molecule, although with different angles between the bonds.
The basicity of phosphine is less than that of ammonia, and unlike ammonia, phosphine is very poorly soluble in water. This is due to the lower electronegativity of phosphorus compared to nitrogen, and therefore phosphorus does not form hydrogen bonds with water. The absence of hydrogen bonds in liquid phosphine leads to the fact that phosphine has a lower boiling point than ammonia, despite the fact that its relative molecular weight is higher.
Phosphorus chlorides
Phosphorus forms two chlorides: phosphorus trichloride and phosphorus pentachloride
Phosphorus trichloride is obtained by passing chlorine over the surface of white phosphorus. In this case, phosphorus burns with a pale green flame, and the resulting phosphorus chloride condenses in the form of a colorless liquid.
Phosphorus trichloride is hydrolyzed with water to form phosphorous acid and hydrogen chloride:
Phosphorus trichloride is used in organic chemistry as a chlorinating agent.
Phosphorus pentachloride can be obtained in the laboratory using the reaction of chlorine and phosphorus trichloride, carried out at a temperature near This reaction is reversible:
Phosphorus pentachloride is a pale yellow crystalline substance consisting of tetrahedral ions and octahedral ions. In a gaseous state it consists of covalent molecules having a bipyramidal shape.
When heated, phosphorus pentachloride dissociates into phosphorus trichloride and chlorine.
Phosphorus pentachloride reacts violently with water to form acid. This reaction is described by the following summary equation:
Phosphorus pentachloride is also used as a chlorinating agent.
Phosphorus oxides
The oxide is a white solid; it is formed during the combustion of white phosphorus in conditions of limited air access. If white phosphorus burns in excess air, another white substance is formed oxide
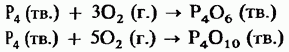
To prevent spontaneous occurrence of these reactions, white phosphorus is stored under a layer of water. The oxide reacts with oxygen when heated to form an oxide
Oxides have acidic properties and "react" with water, forming phosphorous or acid, respectively:
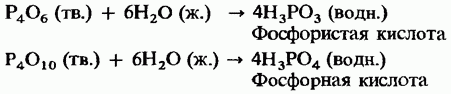
Therefore, both of these oxides must be stored in sealed containers. High water affinity oxide is sometimes used as a drying agent.
Phosphorus Acids and Their Salts
Phosphorus forms a number of oxygen-containing acids (oxoacids). Some and: they are monomeric, for example phosphinic, phosphorous and acids. Phosphorus acids can be monobasic (uniproton) or polybasic (multiproton). In addition, phosphorus also forms polymeric oxoacids. Such acids may have an acyclic or cyclic structure. For example, the acid is dimernuc phosphorus oxoacid.
The most important of all these acids is acid (its other name is phosphoric acid). Under normal conditions, it is a white crystalline substance that diffuses upon absorption of moisture from air. Its aqueous solution is called "syrup-like phosphoric acid." acid is a weak tribasic acid:
Tri- and disubstituted acid salts are usually insoluble in water, with the exception of alkali metal and ammonium salts. Salts containing β-ion have greater solubility. For example, calcium phosphate contained in phosphorus ore is insoluble, and calcium dihydrogen phosphate is soluble. The latter is used as an integral part of superphosphate phosphate fertilizer (see chap. 13).
Phosphinic acid
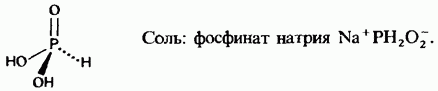
Phosphonic acid
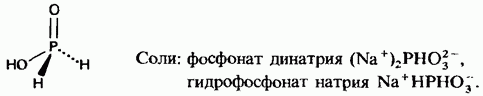
Phosphoric (V) (orthophosphoric) acid
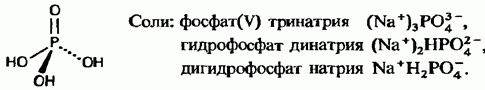
Diphosphoric (V) (pyrophosphoric) acid
Phosphoric (V) acid under industrial conditions is obtained by heating a mixture of phosphoric ore with concentrated sulfuric acid or by dissolving phosphorus (V) oxide in water (see above).
So, repeat again!
1. When moving to the lower part of group V, non-metallic properties of elements are replaced by metal.
2. Phosphorus exists in three allotropic forms.
3. Nitrogen and phosphorus exist in their compounds in oxidation states from -3 to +5.
4. The process of converting atmospheric nitrogen into a form assimilated by plants and animals is called nitrogen fixation. This process is an integral part of the nitrogen cycle in nature.
5. Industrial nitrogen production is based on the liquefaction of air and its subsequent fractional distillation.
6. Phosphorus is necessary for animals to build bone tissue and to provide the body with energy in the process of breathing (see also below).
7. Phosphorus is part of some minerals, the most important of which is apatite.
8. Apatite is used to produce phosphate fertilizers.
9. Ammonia is a covalent compound, the molecules of which have a pyramidal shape.
10. Ammonia has the properties of a Lewis base.
11. Ammonia solutions precipitate insoluble metal hydroxides from solutions of the salts of these metals.
12. Ammonia has the properties of a reducing agent.
13. Ammonium salts decompose upon heating.
14. All six nitric oxides are endothermic compounds.
15. The three most important nitrogen oxides are: a) diazot oxide, b) nitrogen monoxide NO, c) nitrogen dioxide. All of them are simple molecular compounds, which in their electronic structure can be considered as resonant hybrids. for example
16. Nitrogen oxides are considered primary environmental pollutants. In the atmosphere, they enter into various reactions, forming secondary pollutants. The latter can lead to the formation of photochemical smog.
17. Nitric acid is a typical strong acid.
18. Nitric acid is used as a nitrating reagent in organic chemistry.
19. Inorganic nitrates decompose upon heating.
20. For the industrial production of nitric acid, the Ostwald process is used. It consists of three stages: 1) the catalytic oxidation of ammonia, 2) the production of nitrogen dioxide, 3) the conversion of nitrogen dioxide to nitric acid.
21. Both phosphorus chloride are hydrolyzed by water.
22. Both phosphorus oxides have acidic properties.
23. Phosphorus forms a number of oxygen-containing acids (oxoacids). The most important of them is orthophosphoric acid.
PHOSPHORUS(Phosphorus) - a chemical element 15 (Va) of the group of the Periodic system, atomic number 15, atomic mass 30.974. There are 23 known phosphorus isotopes 24 P - 46 P, among them one stable 31 P and only it is found in nature. The half-life of the isotope is 30 P 2.55 minutes; this is the first radioactive isotope obtained artificially in 1934 Frederick and Irene Joliot-Curie.
It is possible that phosphorus in elemental form was obtained as early as the 12th century. the Arab alchemist Alkhid Bekhil during the distillation of urine with clay and lime, this is evidenced by the ancient alchemical manuscript stored in the Paris library. However, the discovery of phosphorus is usually attributed to the ruined Hamburg merchant Hennig Brand. The entrepreneur was engaged in alchemy to get a philosopher's stone and an elixir of youth, with the help of which he could easily improve his financial situation. After evaporation of 50-60 buckets of urine (he took it in the soldiers' barracks) for two weeks and the subsequent strong calcination of the dry residue with coal and sand in a retort, Brand in 1669 was able to condense the released vapors under water and get a small amount of yellow substance. It glowed in the dark and was therefore called Brand “cold fire” (kaltes Feuer). Brand's contemporaries called this substance phosphorus because of its ability to glow in the dark (dr. Greek jwsjoroV). In general, since ancient times, “phosphors” called all substances that can emit light in the dark. So, Bologna phosphorus is widely known - barium sulfide.
In 1682, Brand published the results of his research, and now he is rightly considered the discoverer of element number 15. Phosphorus was the first element, the discovery of which is documented, and its discoverer is known.
The interest in the new substance was tremendous, and Brand took advantage of it - he demonstrated phosphorus only for money or exchanged small amounts of it for gold. Despite numerous efforts, the Hamburg merchant was never able to realize his cherished dream - to get gold from lead using “cold fire”, and therefore he soon sold the recipe for a new substance to a certain Kraft from Dresden for two hundred thalers. The new owner managed to make a much bigger fortune on phosphorus - with "cold fire" he traveled all over Europe and showed it to scientists, high-ranking and even royal people, for example, Robert Boyle , Gottfried Leibniz Karl the Second. Although the method for the preparation of phosphorus was kept a closely guarded secret, in 1682 Robert Boyle managed to get it, but he only announced his methodology at a closed meeting of the Royal Society of London. Boyle's method was made public after his death, in 1692.
For a long time, phosphorus was not considered a simple substance, and only in the 1770s did the French chemist Antoine Laurent Lavoisier in his work on the composition of air, he was able to firmly establish that phosphorus is an elementary substance.
Phosphorus in nature and its industrial production.
The phosphorus content in the earth's crust is estimated at 8 10 -2% by weight. Phosphorus is the eleventh most abundant element on Earth and is one of the twenty most common elements of the solar system. Element No. 15 was found in many types of meteorites (stone and stone-iron) and on the moon. For example, in iron meteorites, the phosphorus content ranges from 0.02-0.94% (mass.), And in various samples of the lunar soil it is 0.05-0.32% (mass.). Despite the fact that geologists classify phosphorus as an impurity element (in the rocks of most of the earth's crust its content is only 0.1%), it is rock-forming, as some rocks are composed almost entirely of phosphate minerals. In the free state, phosphorus does not occur on earth and exists in the lithosphere almost to the highest degree of oxidation, in the form of the orthophosphate ion PO 4 3-. More than two hundred minerals are known containing phosphorus in significant (more than 1%) amounts. Phosphate deposits are usually divided into three groups: apatite deposits, sedimentary phosphorites and guano deposits.
Apatity - a variety of phosphorites, they can be of either magmatic or marine (sedimentary) origin. This name was given to a group of minerals about two hundred years ago, and in Greek it means “deceptive” (ap át án), originally the mineral that was often confused with aquamarine, amethyst or olivine. Apatite minerals are represented by fluorapatite Ca 5 (PO 4) 3 F (most industrially significant), hydroxyapatite Ca 5 (PO 4) 3 (OH) and chlorapatite Ca 5 (PO 4) 3 Cl, francolite (a variety of carbonatapatite) (Ca, H 2 O) 10 (F, OH) 2 (PO 4, CO 3) 6, Wilkeite Ca 10 (OH) 2 (PO 4, SiO 4, SO 4) 6, pyromorphite Pb 10 Cl 2 (PO 4, AsO 4) 6 and many others. The largest deposits of magmatic apatite are in Russia, the countries of South Africa (the alkaline complex of Palabor), Uganda and Brazil. The world's largest magmatic apatite deposit - the Khibiny massif of nepheline syenites - lies on the Kola Peninsula, near Kirovsk. It was opened in 1926 by a group of scientists led by academician A.E. Fersman.
Most of the world's phosphorus reserves marine(sedimentary) phosphorites and their weathering products. They are believed to be of oceanic origin. In the coastal regions of the trade winds, phosphates have been deposited for a long period due to various organic and inorganic processes. The concentration of phosphorites in the field increased as a result of the slow accumulation of phosphates from the environment. The largest deposits of sedimentary phosphorites are owned by Morocco (70% of the world's phosphate reserves) and Western Sahara, USA, China, Tunisia, Kazakhstan.
Guano (Spanish: guano) - natural deposits formed during the decomposition of bones and excrement of seabirds (cormorants, gannets and pelicans), guano deposits sometimes reach one hundred million tons. Guano has been known since time immemorial, as far back as 200 BC. ancient Carthaginians used bird droppings as fertilizer. In the late 19th - early 20th century. the “Bird Islands” of Peru were discovered, so named because of the large number (about 20 million) of seabirds living there. In those days, the Peruvian government earned real income by attracting a large number of tourists to the “Bird Islands” and from the sale of huge quantities of guano as fertilizer. In the last forty years, due to the activities of Peruvian fishermen, the populations of guanoproducing birds have sharply decreased (4 times), so some of the Peruvian “Bird Islands” are now completely empty. The largest deposits of guano are located along the coasts of Africa, South America, California, Seychelles. The highly decomposed guano consists mainly of monetite CaHPO 4 and vitlokite b -Ca 3 (PO 4) 2.
World production (2002) of phosphates is 135 million tons annually. The world's largest phosphate producer is the United States (26% of global production). Development is underway in Florida (Bone Valley Formation), North Carolina, Idaho and Utah. The Kingdom of Morocco (along with Western Sahara) is the second largest producer of phosphate ore (17.3%) and the largest exporter. Phosphorites are developed in three areas: Kurribge, Yussufiya and Ben-Gerir. The main field (Khouribga) is located 120 km south of Casablanca. The total reserves of phosphorites in Morocco are 64 billion tons, explored 10 billion tons (60% of the world's explored reserves). The third largest producer is China (16.7%), and the fourth largest is Russia (10.5%). The main source of phosphorus in Russia is apatite-nepheline ores on the Kola Peninsula. For more than seventy years since the discovery of the field, more than 570 million tons of apatite concentrate have been produced. Now, 10 deposits have been explored within the Khibiny massif, the total reserves of which are 3.6 billion tons, and in general, ore reserves in the Kola Peninsula are about 20 million tons. Given that over the past time, incomplete one and a half billion tons have been mined, the reserves of apatite in Russia should be enough for many more years.
Typically, such a deposit is considered industrial, which produces at least 6,000 tons of phosphate rock per 1 ha. In open pits, phosphate is mined by scraper excavators. First, sand deposits and gangue are removed, and then phosphate ore is recovered. From quarries to concentration plants, ore can be fed (over distances of several kilometers) through steel pipes in the form of water pulp.
In seawater, all inorganic phosphorus is found only in the form of an orthophosphate anion. The average concentration of phosphorus in sea water is very small and amounts to 0.07 mg P / liter. High phosphorus content in the Andaman Islands (about 12 micromol / l). The total oceanic amount of phosphorus is estimated at 9.8 · 10 10 tons.
In the atmosphere of the Earth, phosphorus is completely absent.
Plan
- Phosphorus discovery
- Phosphorus production
- The distribution of phosphorus in nature
- Physical properties
- Chemical properties
- The structure of the phosphorus atom
Phosphorus discovery
The Hamburg merchant Gennig Brand, hoping to improve his financial affairs and avoid complete ruin, decided to try his luck in alchemy. He tried to find "philosopher's Stone" which would make it possible to turn base metals into gold. Mr. Brand got a really happy thought to conduct an experiment with urine. Having evaporated it almost to dryness, G. Brand mixed the remaining substance with coal and sand and heated it in a retort without air. As a result, he received a new substance, which has an amazing property - to glow in the dark.
So in 1669, phosphorus was discovered, which plays an extremely important role in wildlife.
G. Brand was not slow to take advantage of the unusual property of the new substance and began to demonstrate luminous phosphorus to noble persons for a rather high reward. Everything that came into contact with phosphorus acquired the ability to glow. G. Brand deftly used the huge interest of scientists and the general public in phosphorus and began to sell it at a price that exceeded even the cost of gold. Brand kept in the strictest confidence the method of producing phosphorus. None of the other alchemists could get into his laboratory, and therefore many of them began feverishly setting up various experiments, trying to unravel the way to obtain the luminous substance.
Soon a recipe for making"Cold fire" became knownI. Kunkel and K. Kirchmeiro, and in 1680 the secret of phosphorus production was discovered in England by the famous chemist R. Boyle. After the death of R. Boyle, his student, a German A. Gankwitz, made some improvements in the method of obtaining phosphorus, and established its production. It is interesting that A. Gankwitz, despite his long work with phosphorus and very dangerous experiments with it, lived to be eighty years old. He survived his three sons and all those who took part in works related to the early history of phosphorus.
The price of phosphorus since its discovery by I. Kunkel andR. Boyle began to fall rapidly, and in the end, the heirs of the discoverers began to introduce the secret of obtaining phosphorus for 10 thalers.
Getting phosphorus
Phosphorus in industry derived from calcium phosphateCa3 ( PO4 ) 2 , which is isolated from phosphorites and fluorapatites. The production method is based on a reduction reaction.Ca3 ( PO4 ) 2 to phosphorus.
Coke (carbon) is used as a reducing agent for phosphorus compounds. To bind calcium compounds, quartz sand is added to the reaction system.SiO2 . The process is carried out in electric furnaces (production is referred to as electrothermal). The reaction proceeds according to the equation:
2Ca3 (PO4 ) 2 + 6SiO2 + 10C = 6CaSiO3 + P4 + 10CO
The reaction product is white phosphorus. Due to the presence of impurities, technical phosphorus is yellow, so in industry it is calledyellow phosphorus.
The distribution of phosphorus in nature
In the history of chemistry, many great discoveries are associated with phosphorus. However, only a century after the discovery of phosphorus moved from the world of trade and profit into the world of science. Only one event over this long period can be attributed to real science and it has been connected since 1715, when Gensing discovered phosphorus in the brain tissue. This served later for the statement."Without phosphorus, there is no thought."
Yu. Gan found phosphorus in bones in 1769, and two years later the famous Swedish chemist K. Scheele showed that bones consist mainly offrom calcium phosphate, and proposed a method for producing phosphorus from the ash generated by burning bones.
Phosphorus is almost as good as nitrogen in its importance. He participates in the great natural cycle of substances, and, had there not been phosphorus, the flora and fauna would have been completely different. However, phosphorus is not so common in natural conditions, and it accounts for only 0.08% of the mass of the earth's crust. By distribution, it occupies the thirteenth place among other elements. It is interesting to note that in the human body, phosphorus accounts for approximately 1.16%. Of these, 2/3 are in bone tissue, about 0.25% in muscle and about 0.4% in nerve tissue.
Phosphorusfound in nature exclusively in the form of saltsphosphoricacids,the mainwayphosphorite 3 Ca3 ( PO4 ) 2 * Ca( OH) 2 andapatite 3 Ca3 ( PO4 ) 2 * Ca( F, Cl) 2 . Only in certain places are iron phosphates foundvivianite (blue iron ore)Fe 3 ( PO 4 ) 2 * 8 H 2 Oaluminum, for example wavellite 3 Al2 O3 * 2 P2 O5 * 12 H 2 Oas well as rare earths. Phosphoric acid compounds form an essential part of plant and animal organisms. Part of phosphoric acid is bound in them in the form of organic compounds, for example, in the yolk of an egg and in the substance of the brain in the form of lecithins.
Phosphorus is rarely found in large quantities, and in general it should be attributed to scattered elements. In free form, it does not occur in nature, as it is easily oxidized, but is found in many minerals. The main ones are fluorapatite, hydroxylapatite, phosphorite. Somewhat less common are wavianite, monazite, amblygonite, trifilite, and very limited quantities - xenotite and torbornite.
As for the phosphorus minerals, they are divided into primary and secondary. Among the primary, the most common are apatites, which are mainly rocks of igneous origin. The chemical composition of apatite is calcium phosphate, which contains a certain amount of fluoride and calcium chloride. This determines the existence of the minerals fluorapatite and chlorapatite. They contain from 5 to 36% P 2 O 5 . Typically, these minerals are in most cases found in the magma zone, but they are not rarely found in places where igneous rocks come into contact with sedimentary rocks. Of all the known phosphate deposits, the most significant are in Norway and Brazil.
Phosphine and diphosphine are quite rare in nature and often have to deal with phosphorus compounds such as phosphorites. Phosphorites - phosphates of organic origin play a particularly important role in agriculture. On the islands of the Pacific Ocean, in Chile and Peru, they are formed on the basis of bird droppings - guano, which in dry climates accumulates in powerful layers, often exceeding a hundred meters. The formation of phosphorites may also be associated with geological disasters,
for example, with the ice age, when the death of animals was massive. Similar processes are possible in the ocean during the mass death of marine fauna. Phosphorus from organic residues is partially absorbed by plants, but mainly dissolvingin seawater, goes into mineral forms. Sea water contains phosphates in fairly large quantities - 100 - 200 mg / m 3 . With certain chemical changes, phosphates can precipitate and accumulate at the bottom. And when the seabed is raised during certain geological periods, phosphorite deposits appear on land. Similarly, phosphorites of a large domestic phosphorite deposit near Kara-Tau in Kazakhstan could form. Phosphorites are also found in the suburbs.
Physical properties
Phosphorus exists in many versions. However, the “many faces” of phosphorus can be reduced to three main types:white, red andblack.
White phosphorus obtained in the solid state with rapid cooling of phosphorus vapor; its density is 1.83 g / cm 3 . The heat of fusion of white phosphorus is 0.6kcal , and the heat of vaporization 12kcal (per moleP 4 ) In its pure form, white phosphorus is completely colorless and transparent; It is usually sold in molded sticks that are easily cut with a knife. This operation must be done under water (preferably at 20-25 about C), since when cut in air, phosphorus can ignite from friction. In cold white phosphorus is brittle, but at temperatures above 15 about C becomes soft.
White phosphorus oxidizes very rapidly in the air and glows in the dark. Hence the name "phosphorus", which in Greek means "luminiferous". Already with weak heating, for which simple friction is enough, phosphorus ignites and burns, emitting a large amount of heat. It burns with a yellowish-white flame, forming pentoxide: 2P + 5 / 2 O 2 = P 2 O 5 + 370 kcal Phosphorus can also ignite in air due to the release of heat during oxidation. To protect white phosphorus from oxidation, it is stored under water. White phosphorus is insoluble in water; dissolves well in carbon disulfide. Its solubility in carbon disulfide is extremely high (about 10: 1 under normal conditions).
White phosphorus - strong poison. A dose of 0.1 g is fatal to humans.
If white phosphorus is heated for a long time without air access at a temperature of 250 - 300 about C, it turns into another phosphorus interaction, which has a red - violet color and is called red phosphorus. The same transformation occurs, but only very slowly, under the action of light.
Red phosphorus its properties are very different from white: it oxidizes very slowly in air, does not glow in the dark, it lights up only at 260 about C, does not dissolve in carbon disulfide and is non-toxic. The density of red phosphorus is 2.0-2.4 g / cm 3 . The variable density value is due to the fact that red phosphorus consists of several forms.
With strong heating, red fossfor, without melting, evaporates (sublimates). Cooling down the vapor produces white phosphorus.
Black phosphorus formed from white when heated to 200-220 about With under very high pressure. It looks like graphite, feels greasy and heavier than other modifications; its density is 2.7 g / m 3 . Black phosphorus has semiconductor conductivity (with a band gap of 0.33at) . Under pressure of 18 thousand atm. Black phosphorus melts around 1000 about C, and under the pressure of only its steam above 550 about C becomes purple.
Chemical properties
1. The connection of phosphorus with hydrogen and halogens . Phosphorus forms gaseous hydrogenhydrogen phosphide, orphosphine, PH 3 . It can be obtained by boiling white phosphorus with an alkali solution or by the action of hydrochloric acid on calcium phosphide.Ca 3 P 2 :
The structure of the phosphorus atom
The structure of the outer electron layer of the phosphorus atom 3s 2 p 3
Atomic ionization energy, eV 10.49.
Relative electronegativity2,2.
The phosphorus atom nucleus charge is +15 ( +15 P)
Atomic radius, nm 0.13