The use of surfactants. Olloid surfactants. Surfactants. Natural and synthetic. Their advantages and disadvantages
All soluble substances by their ability to adsorb at the liquid-air interface can be divided into two groups: surface-active substances and surface-inactive substances. Surfactants able to accumulate in the surface layer. This phenomenon is called positive adsorption. Positive adsorption occurs if: - the surface tension of the solute is less than the surface tension of the solvent (in this case, the free surface energy decreases); - the solubility of the substance is relatively low. Surfactants include fatty acids with a sufficiently large hydrocarbon radical, salts of these acids (soaps), sulfonic acids and their salts, high molecular weight alcohols, amines. A characteristic feature of the structure of all surfactants is their diphilicity, i.e. that they consist of two parts - the polar group and the non-polar hydrocarbon radical. The polar group determines the affinity of the substance for water, and the hydrophobic hydrocarbon radical causes a reduced solubility in water. Surface inactive substances tend to leave the surface in the volume of fluid. This phenomenon is called negative adsorption. They have good solubility in water and higher surface tension. Surfactants include all inorganic electrolytes - acids, alkalis, salts. Substances whose surface tension is equal to the surface tension of the solvent are evenly distributed between the surface layer and the volume of the solution. Such substances include sugar. The surface activity of a substance depends only on its nature, but also on the nature of the solvent. Water has a large surface tension, and therefore, many substances exhibit surface activity with respect to it. Alcohol has a significantly lower surface tension than water. Therefore, some substances that are surface active with respect to water are inactive with respect to alcohol. Many diphilic surfactants can form both true and colloidal solutions. For such systems, there is a reversible transition and the corresponding thermodynamic equilibrium true solution ↔ sol ↔ gel. The systems in which such transitions are observed include aqueous solutions of soaps, tannides (tannins), some dyes, and others. Solutions of colloidal surfactants form spontaneously, at low concentrations they are molecular, and micelles from diphilic surfactant molecules appear in them at higher concentrations in equilibrium with a molecular solution of constant and low concentration. The appearance of micelles in solution occurs when a certain concentration, called critical micelle concentration (CMC) . A change in the structure of the solution during CMC leads to a sharp change in the physicochemical properties of the solution. The micelle formation mechanism is related to the surfactant adsorption mechanism: the interaction forces between water molecules are greater than between water molecules and surfactants; surfactant molecules are first pushed out of the water into the surface layer, where they are adsorbed and oriented by hydrocarbon chains into a non-polar medium. Then, with increasing concentration, surfactant molecules are pushed out by water molecules into micelles, and, seeking to find a favorable orientation, they turn to each other with their non-polar hydrocarbon chains - spherical micelles arise. With an increase in the surfactant concentration in the solution, spherical micelles are rearranged into rod-shaped and then lamellar ones. Micelle formation leads to an increase in the viscosity of the system up to the loss of fluidity - the sol turns into a gel. The systems formed spontaneously with equilibrium molecularly and colloidal parts are called lyophilic colloidal systems . Lyophilic colloidal systems are characterized by solubilization , which consists in the ability of water-insoluble organic substances to dissolve in the hydrocarbon part of surfactant micelles. As a result, almost transparent, thermodynamically equilibrium solutions are formed. The solubility of organic substances increases with increasing molecular weight of the surfactant, the concentration of surfactants, in the presence of electrolytes that contribute to micelle formation. Organic substances with a small molecular weight and containing polar groups are more easily solubilized. The phenomenon of solubilization is extremely important for the polymerization of unsaturated hydrocarbons in emulsions in order to obtain synthetic latexes or synthetic rubbers. Reverse solubilization - the dissolution of water in oils in the presence of appropriate colloidal surfactants dissolved in oil is of great importance in the food industry, in particular in margarine production. Colloidal surfactants are of great practical importance. They are used, for example: - as stabilizers of disperse systems; - to change the nature of the surface (hydrophobization or hydrophilization); - to reduce crushing strength; - are one of the main components of lubricants; - as components of detergents.
As surfactant monomers are added to the solution, the surface or interfacial tension will decrease until the surfactant concentration reaches the so-called critical micelle concentration. In addition, the aggregate structure is dictated by the polarity of the solvent in which the surfactant is dissolved. For example, in an aqueous solution, groups of polar heads of micelles will be oriented outward to the aqueous phase, and hydrophobic tails will bind in the micelle core.
In contrast, in the oil, the polar head groups will be associated at the center of the micelle, while the hydrophobic tails will be oriented externally. Although many common cellular components are surface active, such as fatty acids and phospholipids, a large number of microorganisms produce certain biosurfactant molecules that have unique structures. The earliest interest in biosurfactants arose as a result of their antibiotic activity. Of further interest was the observation that biosurfactants were obtained in response to the presence of hydrophobic substrates, which indicates the possibility of their use in the treatment of oil waste and in oil production.
Soaps and synthetic detergents
Conventional soaps are salts of monobasic carboxylic acids with a long hydrocarbon chain (C 15 - C 22). For technical purposes, sodium salts of palmitic, stearic and unsaturated oleic acids are of particular importance; potassium and ammonium salts, liquid under ordinary conditions, are of less importance. Salts of divalent and trivalent cations (Ca +2, Mg +2, Al +3, etc.) are insoluble in water, but form colloidal solutions in hydrocarbon media and are used in greases in mineral oil, as well as for stabilization of emulsions of the second kind. The salts of naphthenic acids contained in soap oil, a product obtained by refining kerosene and hydrochloric oil, also have a washing effect. Detergents include salts of sulfonic acids containing a sulfo group as an active group (alkyl sulfonates C n H 2n + 1 SO 3 Me and alkylaryl sulfonates C n H 2n + 1 C 6 H 4 SO 3 Me, where Me is a monovalent cation of sodium, potassium, ammonium ) Alkyl sulfates - sulfuric acid esters with higher alcohols, as well as their salts C n H 2n + 1 - O - SO 3 Me, are also widely used as detergents. Since sulfonic acids are strong acids, not only their salts with monovalent cations, but also salts with multivalent cations, as well as the sulfonic acids themselves are quite soluble in water. This is an important advantage of sulfo-soap over conventional soaps, which allows you to use them as detergents in hard and acidic water. In the salts of carboxylic and sulfonic acids, the anion is the carrier of surface-active properties. It is the anion that is adsorbed on the surface, as a result of which the surface is negatively charged. Such surfactants are called anionic . If the cation is a carrier of surface-active properties, then surfactants are called cationic . Such soaps include salts of tetra-substituted ammonium bases, for example, C 16 H 33 (CH 3) 3 NCl cetyltrimethylammonium chloride, C 18 H 37 NH 2 HCl octadecylammonium chloride, pyridine compounds substituted at the nitrogen atom, for example cetylpyridinium chloride, etc. There are also non-inogenic soaps unable to dissociate in solution. Their molecules usually consist of a long hydrocarbon chain with several polar, but nonionic groups at the end, which determine the solubility of these soaps. Such groups are hydroxyl or ether groups. An example of such compounds is the product of the interaction of high molecular weight alcohol or alkyl phenol with several molecules of ethylene oxide. C n H 2n + 1 (OCH 2 CH 2) m OH. The washing action of soaps is associated with a number of different effects: - Soaps lower the surface tension of the solution, thereby wetting the fabric with a washing liquid. This facilitates the penetration of liquid into such capillaries of contaminated tissue into which pure water cannot penetrate. - Soap molecules create a well-hydrated adsorption layer on the surface of the fiber and particles of solid and liquid contaminants, which causes a wedging pressure. This contributes to the separation of pollution particles from the surface of the fiber. - Adsorption films on the surface of the particles give these particles high aggregate stability and prevents them from sticking to the surface of the fiber elsewhere. - In the presence of soap, a foam is formed that promotes mechanical entrainment of contaminants. - If the particles of impurities are oily in nature, they can be solubilized in soapy solutions.
Interest in biosurfactants remains high because it is considered environmentally compatible. Biosurfactants are substances produced by microorganisms with a tendency to accumulate at interfaces, especially at liquid-air boundaries. These compounds inhibit the growth of pathogens, avoiding the adhesion of microorganisms along epithelial cells.
These substances include various chemical structures, such as glycolipids, lipopeptides, polysaccharide-protein complexes, phospholipids, fatty acids and neutral lipids. In addition to this inhibitory ability, biosurfactants also help the binding of lactobacilli to collagen on cells. Biosurfactants exhibit various advantages over chemical surfactants: they have low toxicity, high biodegradability and high efficiency at extreme temperatures or pH values.
Surfactants
1. Surfactants include substances that can reduce surface tension, because σ Pav< σ жидкости. Поверхностно-активными по отношению к воде являются вещества менее полярные, чем вода (спирты, амины, жирные кислоты, мыла, белки и др.)
2. For them, dσ / dС< 0, т.е. с увеличением концентрации ПАВ поверхностное натяжение раствора уменьшается. Графически эта зависимость изображается кривой - изотермой поверхностного натяжения (Рис. 4). Из графика видно, что для ПАВ характерно резкое снижение σ даже при малых концентрациях. По мере роста концентрации ПАВ график становится более пологим и, наконец, переходит в горизонтальную прямую. Это означает, что поверхностное натяжение достигло своего минимального значения. При этих условиях на поверхности жидкости образуется сплошной мономолекулярный слой ПАВ и дальнейшая адсорбция уже невозможна.
Surfactants, also known as surfactants, are those substances that are predominantly adsorbed at the air-liquid, liquid-liquid or liquid-solid boundaries. The surface activity of a solute refers to a particular solvent. Molecules of surfactants contain at least two separate parts, a part that interacts strongly with a solvent, a lyophilic part, and another fragment of a lyophobic part, the interaction of which with a solvent is less than its interaction with molecules of a similar structure.
Fig. 4 surface tension isotherm for surfactant
3. In 1878, the American scientist J. Gibbs deduced an equation relating the amount of adsorption of a substance (G) with its ability to change the surface tension of a solution (dσ / dС)
![]()
Where C is the concentration, mol / l
R is the universal gas constant of 8.32 J / mol · K
This part of the molecule gives solubility to the molecule, while the lyophobic part limits or even prevents the solution. In systems with oil water, the hydrophobic group is usually a hydrocarbon with a single or branched chain containing 8-18 carbon atoms, the solubility of the molecule decreases with increasing chain length.
The number originally proposed by Davis is an empirical and convenient measure widely used for composition. The group numbers are shown in table 1. Anionic surfactants are the most common and are used mainly as detergents. Positively charged cations have greater bacterial affinity and are used in medical applications and cosmetics. They are now easily adsorbed to negatively charged textile fibers and are effective softeners and conditioners.
T - absolute temperature, K
dσ / dС - change in surface tension with concentration at a constant surface value.
4. It follows from the Gibbs equation that for dσ / dС< 0 величина Г > 0. They say that surfactants are characterized by positive adsorption, when the surface concentration of surfactant molecules is greater than in volume.
5. Surfactant molecules have a diphilic structure: they contain polar and nonpolar groups. The atomic groups such as —COOH, —OH, —NH 2, —NO 2, —SO 2 OH, and others possess polar properties. They are capable of hydration and are hydrophilic. The nonpolar part of the surfactant molecules is a hydrophobic hydrocarbon chain or an aromatic radical. Thus, alcohols, amines, fatty acids, soaps, proteins, etc. are surface active with respect to water.
Nonionic surfactants are increasingly used as dispersing agents in the water paint industry, in emulsion technology and in the rheological behavior of pastes, sludges and drilling fluids. Interphase phenomena, Academic Press, New York. Systematic analysis of surface-active agents, chemical analysis, vol. 12, 2nd ed. John Wiley and Sons. Lowering liquids or biphasic systems.
The structural commonality of most surfactants is the presence of at least one hydrophobic and at least one hydrophilic region. Due to differences in chain length in many surfactants, one often speaks of a hydrophilic “head” and a hydrophobic “tail”.
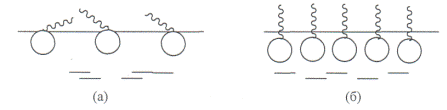
6. Due to the diphilic structure of surfactants, their molecules spontaneously form an oriented monolayer at the interface: the polar groups of molecules are located in the aqueous (polar) phase, and the hydrophobic radicals are in the less polar phase. The reason for this orientation is that the energy of interaction of water molecules with each other is greater than with the hydrophobic parts of surfactant molecules: En 2 about - n 2 about\u003e En 2 about - pav. For the image of surfactant molecules, conventions are used. A straight or wavy line indicates a hydrocarbon radical, and a circle indicates a polar group. Schematically, the orientation of surfactant molecules can be represented as follows (Fig. 5).
During adsorption at the interface, a region with a low affinity for the surrounding phase appears outside the interface, and the region with greater affinity coincides with the bulk phase. Surfactants are most commonly used in water as the surrounding phase, so that the hydrophobic group is directed outward.
Ampholytic surfactants: compounds having an anionic and cationic group; often carboxylate and quaternary amino groups. Non-ionic surfactants: Compounds with non-ionic polar groups such as alcohol, ether or ethoxylate. Quaternary amines. . Surface tension is due to the fact that for liquid molecules, staying in the bulk phase is energetically preferable compared to staying at the interface or interface. In contrast, surface surfactants are more preferable in structure at the interface, so that in the presence of surfactants, the labor force necessary to form a surface is reduced.
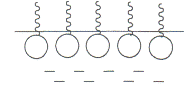
Fig. 5 Orientation of surfactants at the liquid-gas interface
7. The ability of a surfactant to reduce surface tension is quantified by the surface activity q \u003d - dσ / dС. In homologous series, clear patterns are observed in the change in surface activity: it increases with increasing length of the hydrocarbon radical and depends on the non-polarity of the substance. At the end of the 19th century, Duclos and Traube formulated a rule on the basis of large experimental material: with an increase in the length of the hydrocarbon chain by the —CH 2 group, the surface activity increases by 3–3.5 times. In other words, an increase in chain length in arithmetic progression leads to an increase in surface activity exponentially. In fig. Figure 6 shows the surface tension isotherms for a number of acids. The graph shows that q 1 When the surface is completely filled with molecules of surfactants in the bulk phase, so-called aggregates are formed. They are used wherever it is necessary to improve contact between the various phases or to mix them: during cleaning and coating during emulsification, dispersion or flooding. Measuring the effect of the addition of a surfactant on the wetting of solid surfaces, we measure it. If the detached oil droplets and dirt particles were not suspended in the detergent solution in a stable and highly dispersed state, they would be inclined or merged into aggregates large enough so that they could be re-deposited on the cleaned surface. When washing fabrics and similar materials, small droplets of oil or small, deflocculated dirt particles are easier to carry through gaps in the material than relatively large ones. Therefore, the action of the detergent while maintaining the dirt in a finely divided state is important in order to prevent the cloth from retaining the separated dirt. Fig. 6 Isotherms of the surface tension of certain acids: 1. CH 3 COOH - acetic acid, 2. CH 3 CH 2 COOH - propionic acid, 3. CH 3 (CH 2) 2 COOH - butyric acid, 4. CH 3 (CH 2) 3 COOH - isovaleric acid. The rule is valid for aqueous solutions and applies to hydrocarbon media. Indeed, the longer the hydrocarbon chain, the non-polar substance, the more its molecules are pushed to the surface by water, because E n 2 o-2 o\u003e E n 2 o-pav. The Duclos-Traube rule was the theoretical basis for the synthesis of modern detergents. For use as detergents, soap and detergents must have certain chemical structures: their molecules must contain a hydrophobic moiety, such as a long chain carbon group or, for example, alkylbenzene. This hydrophilic moiety makes the molecule soluble in water. In general, the hydrophobic part of the molecule attaches to the solid or fiber and to the soil, and the hydrophilic part attaches to water. There are four groups of surfactants. Which produce electrically negative colloidal ions in solution. which produce electrically positive ions in solution. which produce electrically neutral colloidal particles in solution. or amphoteric, detergents that are capable of acting as anionic or cationic detergents in solution, depending on the pH of the solution. The first detergent was soap. In a strictly chemical sense, any compound formed by the reaction of a water-insoluble fatty acid with organic or a can be called soap. 8. In accordance with the Duclo-Traube rule, adsorption increases with chain elongation in the homologous series, but for all members of the series the adsorption value tends to the same limiting value Г ∞, called limiting adsorption (Fig. 7). Fig. 7 A series of adsorption isotherms at the solution-gas interface for the homologous series of surfactants. 1 - for the lowest member of the row, 3 - for the highest member of the row In practice, however, the soap mainly concerns those water-soluble soaps that result from the interaction between fatty acids and alkali metals. In some cases, however, salts of fatty acids with ammonia or with triethanolamine are also used, as in shaving preparations. According to the Phoenicians, they prepared it from goat and ashen in 600 BC. and sometimes used it as a subject of barter with galls. Soap was widely known in the Roman Empire; whether the Romans learned about its use and manufacture from the ancient Mediterranean peoples or from the inhabitants of Britain is unknown. The importance of soap for washing and cleaning did not seem to be recognized until the 2nd century; Greek physician Galen mentions it as a and as a means of cleansing the body. Earlier soap was used as. The presence of Г ∞ is evidence of the existence of a monomolecular surfactant layer on the liquid surface. At low concentrations in a region far from saturation, hydrocarbon chains pushed into the air “float” on the surface of the water, while the polar group is immersed in water. The interaction between surfactant molecules is insignificant; monolayers are called gaseous (Fig. 8a). With increasing concentration, the number of molecules in the surface layer increases, the chains rise and, in the limit, acquire a vertical position (Fig. 8b). In works written by an 8th-century Arab scholar, Jabir ibn Hayyan, soap is repeatedly mentioned as a cleanser. In Europe, soap production in the Middle Ages focused first on Marseille, later in Genoa, then in Venice. Leo, sent Lady von Schleinitz, a parcel with soap from Italy, he accompanied her with a detailed description of how to use the mysterious product. The Celts used animal fats containing a percentage of free fatty acids. The presence of free fatty acids certainly helped start the process. This method probably prevailed until the end of the Middle Ages, when slaked lime was used to causticize alkali metal carbonate. Through this process, chemically neutral fats can be easily saponified with caustic liquor. Fig. 8 Scheme of the formation of a monomolecular layer With this orientation, a change in the chain length does not change the area occupied by the molecule in the surface layer, and, therefore, does not change the number of molecules per unit surface proportional to Г ∞. Such monolayers are called condensed. It is known that the use of solubilizers in principle is associated with a number of problems and inconveniences. In addition, the strongest solvents are harmful or even dangerous to humans and the environment and can damage a cleaned surface. Our soil-releasing substances are free from many of the disadvantages inherent in traditional solvents and can replace almost all of them. They are absolutely safe for all materials, do not cause corrosion and corrosion of metals, do not pollute the environment. They easily remove all types of dirt. Surfactants make water more liquid, which allows our products to penetrate the layer of dirt. Molecules of surfactants include microparticles of oils, separating them from each other and contributing to the degreasing. The same removes mold and mildew from surfaces without the residual and unpleasant odor of chlorine. One of the challenges for the future is the creation of products that are less hazardous, less polluting, and require less energy. One of these products is an enzyme. 9. According to the ability of molecules to dissociate into ions, surfactants are divided into two large classes: ionic (dissociative) and nonionic (non-dissociative). Ionogenic surfactants, in turn, are classified into 1) anionic, giving a surface-active anion upon dissociation: RCOOMe soaps (Me - K +, Na +, NH 4 +), sulfonic acids, their salts and other compounds; 2) cationic, forming a surface-active cation upon dissociation: salts of amines, quaternary ammonium bases, alkyl pyridine compounds; 3) amphoteric, capable of depending on pH to exhibit anionic properties (in an alkaline environment) or cationic properties (in an acidic environment): alkyl amino acids, etc. As an example of anionic surfactants used in medicine, sodium lauryl sulfate - Na +, cationic surfactants - cetyltrimethylammonium bromide + Br - can be cited. Amphoteric is alkyl diaminoethyl glycine hydrochloride HCl. Cationic and anionic surfactants are used in surgery as antiseptics. For example, quaternary ammonium compounds are approximately 300 times more effective than phenol in the destructive effect against microorganisms. When the length of the alkyl radical from C 8 to C 14 surfactants have a pronounced antiphage activity. The antimicrobial effect of surfactants is associated with their effect during adsorption on the permeability of cell membranes, as well as the inhibitory effect on the enzyme systems of microorganisms. Nonionic surfactants are obtained by the interaction of higher alcohols, acids or phenols with ethylene oxide molecules. Compounds of the type R (OCH 2 CH 2) m OH are obtained. The longer the oxyethylene chain, the more pronounced the hydrophilic properties. They are widely used in pharmacy in the role of stabilizers of spana and tween (esters of fatty acids, sorbitol, or ethoxylated sorbitol)