The facilities are lower quality pavas. Phosphate Powders - Health Harm
Household chemicalsTo which various detergents and disinfectants belong, tops the list of our daily necessities. But do not forget that everything has a downside: in the struggle for cleanliness, we can cause irreparable harm to both the surrounding nature and our health. An example of this is washing powder with phosphates.
The composition of modern washing powders includes more than 20 components, which are often hazardous chemical compounds: surface-active substances (surfactants), emulsifiers of fats (phosphates), alkalis (phosphates of alkali metals, soda) and other active ingredients (chemical and optical bleaches), substances for binding ions of magnesium and calcium (sodium tripolyphosphate) and odorants.
Dangerous components of washing powders with phosphates and other harmful substances can enter our body through the skin of our hands and body (when we wash with hands without protective gloves) through water contaminated with drains after washing (even cleaning with the most powerful filters does not give the desired results) and through the respiratory tract.
What is so harmful these substances? About it Great Epoch told the environmental organization "MAMA-86".
Surfactant:
- cause dysfunction and integrity of cells, accumulate in the organs of the body;
- The action of surfactants is similar to the action of some poisons: they cause an effect in the lungs, which in turn leads to an increase in cholesterol levels;
- alter the physico-chemical parameters of the blood and harm the immune system;
- cause allergies;
- increase the risk of infertility.
Phosphates, phosphonates:
- significantly enhance the toxic properties of a-surfactant and their penetration into the body;
- contribute to the defatting of the skin, the active destruction of cell membranes, reduce the barrier function of the skin;
- can cause allergies and dermatosis;
- upon contact with the skin they enter the blood, changing the percentage ratio of hemoglobin, protein, structure and density of blood serum, which leads to disruption of the internal organs, causes a metabolic disorder, exacerbation of chronic diseases and the emergence of new ones.
In synthetic detergents, phosphates are used to bind calcium and magnesium ions. Once in the environment, they cause accelerated development of algae in water bodies. Toxins released by blue-green algae are dangerous to aquatic animals. In addition, blue-green algae worsen the drinking quality of water, water acquires different smells and tastes.
Such polluted water containing cyanobacterial toxins also causes the development of cancer cells, problems during pregnancy, congenital injuries, increased morbidity and reduced overall life expectancy.
For over 20 years, the use of phosphates in laundry detergents has been banned in many countries.
Anionic Surfactants (a-surfactant)
Cheap in production and well soluble, but very dangerous for the body and nature.
Anionic surfactants are able to accumulate in organisms in significant concentrations, which reduces immunity, causes allergies, affects the brain, causes kidney, liver and lung diseases.
Cationic Surfactants
They have bactericidal properties, but also affect the body and nature, although to a lesser extent than a-surfactant.
Nonionic surfactants
Like any "chemistry" also affect the body and nature. But their advantage is 100% decomposition.
Other impurities
The mechanisms by which impurities affect the body are still being investigated. Already there is evidence that some of them have a negative impact on metabolic processes.
How to choose a safe washing powder?
1. If the package does not contain data on the composition of detergent, it is not safe to use it.2. When choosing a powder, carefully read the inscriptions on the package, paying attention to the quantitative composition of the components, first of all, surfactants and phosphates (phosphonates are just as harmful as phosphates; in some powders it says “phosphate free”, but there are phosphonates) .
3. Choose those powders that contain the least amount of surfactants and phosphates.
4. Choose everyday chemical goods without a distinct aroma, especially for washing baby clothes.
5. If possible, instead of washing powder, buy and use alternative detergents, such as laundry soap, soda.
Take care of your health and the environment!
Based on materials of MEA-86.
The most commonly used surfactants for skin cleansing are the main active ingredients of detergents, and the strength of the agent’s effect (mild or irritant) depends on them. Basically, when creating detergents, anionic (negatively charged) surfactants are used as surfactants, as they have the best ability to foam and soap. Since the detergents in the form of briquettes are hard because of the need to preserve the shape and structure, which should not change during the production process, it is possible to use only certain surfactants. In the manufacture of liquid detergents, on the contrary, there is a larger selection of active chemical components. In addition, the production process of liquid detergents is such that emollients can be included in them in larger volumes than in solid detergents. Structural formulas of surfactants most often used in the creation of solid and liquid detergents.
Surfactants in solid detergents
The main surfactant found in most solid detergents worldwide is soap (alkyl carboxylate). Soaps, also called natural surfactants, are usually formed during the saponification of fats, during which an interaction occurs between the triglyceride and the alkaline compound. In the production of soap, vegetable oils are commonly used, such as palm oil, palm oil derivatives (palm stearin, palm olein), rice, peanut or castor oil in combination with coconut or palm oil. The components of non-plant origin used to create soap are usually made from animal fats, such as rendered lower grade fats. Although soaps are effective as cleansers, they negatively affect the condition of the skin. The use of soap, especially in countries with a cold climate, is associated with the appearance of erythema, xerosis and itchy skin.

The ability of detergents to irritate the skin depends on several factors. The type of surfactant used is critical. Surfactants with a molecular chain length from C8 to C14 are the most active components in solution, so they have the most pronounced irritant effect. The composition of detergents on a soap base usually includes these surfactants. Irritant effect is also due to the fact that these detergents are poorly washed off the skin (while surfactants may remain on the surface) and increase its pH. If the pH of the skin remains elevated for more than 4 hours (for example, when using alkalinity increasing agents, or when washing the skin frequently), the alkalinity index on the surface of the skin increases, which can lead to irritation. The pH value for most soaps ranges from 9.5 to 11.0, which is typical of an alkaline environment. As a result of attempts to reduce the irritant effect of soaping agents by adding additional components to their composition, new types of soaping agents have been developed, such as perezhirayuschie additives, transparent soaps and combined solid soaps.
Soap with perezhirivayuschimi additives.
These soaps are obtained by incomplete saponification (neutralization), during which fatty acids or oils do not react. They can also be synthesized by adding to the soap during the production of fatty alcohols, acids or esters. Usually due to perezhirivayuschih additives improve the properties of soaping means, such as:
softness of action;
the ability to moisturize the skin;
foaming ability;
the expense of means decreases.
Transparent soaps.
The composition of these funds in high concentrations contain moisturizers, such as glycerin. Thanks to these moisturizers that increase the solubility of the soap, it becomes transparent. However, these products are characterized by a high content of active soaping substances and an alkaline pH, which increases their irritant effect. However, transparent soaps are classified as mild soaps, due to the presence of glycerin, which is a moisturizer, and a low content of fatty substances.
Combined solid soaps.

These products usually contain natural soaps and mildly acting synthetic surfactants. Synthetic surfactants reduce the irritant effect of these agents, however, the pH at the same time remains high, approximately at the level of 9.0-9.5. Combined solid soaps are usually less likely to cause skin irritation than standard solid soaps.
Solid synthetic detergents.
Solid soaps consisting of surfactants are called solid synthetic detergents. In contrast to soaping agents, solid synthetic detergents are obtained by esterification, ethoxylation and sulfonation of oils, fats or petroleum derivatives. Solid synthetic detergents typically contain the following synthetic surfactants:
sulfosuccinates;
cocoyl sulfate sodium monoglyceride;
sodium cocoyl isethionate.
alkyl glycerol sulfated esters;
a-olefin sulfonates;
Solid cleansing soaps (containing alkylcarboxylate) have an alkaline pH, the values of which range from 10 to 10.5. In contrast, solid synthetic detergents (the main substance alkyl isethionate) have a neutral pH. Additional components of solid synthetic detergents are refractory fatty acids, waxes and esters. It should be noted that sodium cocoyl isethionate, the most commonly used synthetic surfactant, has particular molecular properties that were used in the development of a new direction in the production of detergents, namely, in the creation of mildly active substances.

Surfactants most commonly used in the manufacture of liquid detergents
The composition of liquid detergents usually simultaneously includes anionic and amphoteric (containing a neutral charge) surfactant. Non-ionic surfactants and surfactants, synthesized from amino acids, are more and more often included in the composition of detergents, as they provide a soft action. Anionic surfactants commonly found in liquid detergents include soaps (salts of fatty acids) and synthetic surfactants, such as:
alkyl ether sulfate;
alkylaryl isethionates;
alkyl phosphates;
alkyl sulfosuccinates;
alkyl sulfonates.
In the production of liquid detergents, anionic surfactants derived from amino acids (for example, acylglycinates) are most often used as the main surfactants. The most commonly used zwitterionic surfactants are cocamidopropyl betaine and cocoamphoacetate. One of the non-ionic surfactants that make up some detergents is alkyl polyglycoside. Surfactants derived from amino acids, such as alkyl glutamates, sarcosinates and glycinates, are increasingly used in the manufacture of detergents. Most liquid detergents are characterized by a neutral or acidic pH. The exceptions are the means for creating which soap (alkyl carboxylates) is used as the main active ingredient. The pH of these detergents is alkaline.
Other components included in the composition of the skin wash
In addition to surfactants, detergents contain structuring substances, organoleptic properties modifiers and flavors. Flavoring agents may be the most expensive component of the detergent, but the importance of their presence for consumers cannot be overestimated. In solid detergents, structuring substances are needed to maintain their “solid state” and to facilitate a rather complex production process. Most often, long-chain fatty acids, waxes and alkyl ethers are used as structuring agents. In the production of liquid detergents, structuring substances are used in order to provide the necessary rheological properties / density of the liquid, which affect the flow rate and the particular application of the product. In addition, structuring substances provide physical stability of dispersion-suspension systems and the presence of a moisturizing effect. Softeners are included in the detergent composition in order to achieve a minimum drying effect on the part of the surfactant. Triglyceride oils, fats, liquid paraffin, waxes and mineral oils are used most often in the manufacture of moisturizing shower gels as softening / occlusive agents. To enhance the moisturizing effect, water-soluble moisturizers, such as glycerin, can also be added to the products.

Detergents designed to produce special effects may contain other additional active ingredients. For example, antimicrobial detergents often contain bactericidal agents, such as triclosan or triclocarban. The list of additional active ingredients used to achieve special additional effects is established by the FDA. The FDA controls the safety of detergents and detergents that have an antibacterial effect or other effects similar to those of drugs. The safety of true soaps that do not have other effects is controlled by the Consumer Product Safety Commission. Detergents developed for frequent disinfection of the skin of the hands of health care workers or the food industry should comply with more stringent requirements than the above products. These agents usually contain potent cationic antimicrobial agents, such as chlorhexidine or benzalkonium chloride. Additional components, such as salicylic acid or benzoyl peroxide, are also included in facial cleansers designed to treat acne. Facial cleansers are usually characterized by a relatively low pH. With the development of technologies for the production of detergents, thanks to which it was possible to achieve a positive effect of these funds on the condition of the skin, they began to include additional components, such as nutrients and substances that reduce the severity of skin aging.
Hello connoisseurs of beautiful and eco-friendly! My name is Svetlana, and this is my first review of such a plan on this site. I probably have been interested in environmental beauty products and home cleaning for a year now. And in general, as the year began to pay more attention to themselves and their health.
And of course, once I had a question, or rather I wanted to know more about what I clean the house with, in particular, how I wash the dishes. When I indicated on the Internet the names of the components that make up “my product” with which I was washing dishes at that time, and read what this component was and what it was made of, my hair quietly stood on end. Of the 10 ingredients on the tool as I remember it PRIL, one was of plant origin, everything else is synthetic. . And from that moment on I began to pay more attention to what I wash the dishes with and how I clean the house. From that moment I began to search more information on the Internet, such as finding out what was happening, watching bloggers who realized the harm we cause to our body with these means earlier than me, and already have some experience in this matter. So I stumbled upon ecoville. The first tool I ordered for washing dishes was Concentrated liquid product for washing dishes "Granat" Sodasan. The composition can be read by following the link. I used until I learned that anionic surfactants contained in this tool are dangerous to health.
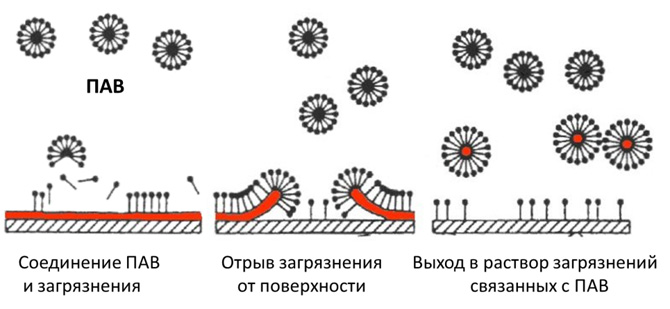
Surfactant (Superficially Active Substances) - these are usually chemicals that are contained in any detergent, even in regular soap. Just thanks to surfactants cleaning agent cleans. What are surfactants for?
The problem is that dirt, especially grease, is very difficult to wash off with water. Try to wash greasy hands with water. Water will drain without washing off the fat. Water molecules do not stick to fat molecules and do not take them with them. Therefore, the task is to attach fat molecules to water molecules. This is exactly what surfactants do. The surfactant molecule is a sphere, one pole of which is lipophilic (it combines with fats), and the other is hydrophilic (it comes into contact with water molecules). That is, one end of the surfactant particle is attached to the fat particle, and the other end to water particles.
Anionic Surfactants - The main advantage is the relatively low cost, efficiency and good solubility. But they are the most aggressive towards the human body. Cationic surfactants have a bactericidal property.
Nonionic surfactants - The main advantage is a favorable effect on the fabric and the main thing - 100% biodegradability.
Ampholytic surfactants - depending on the environment (acidity / alkalinity), they manifest themselves either as cationic or as anionic surfactants.
Vegetable safe surfactants (sugar surfactants)
Of course, I didn’t order more medicine from Sodosan, and began to look for something without anionic surfactants but on sugar surfactants. I met a girl at one meeting, she was a distributor of Siberian Health. And I took two tests from her. One of them is dishwashing detergent. Greenpin ECO Dishwashing Detergent
Dishwashing detergent with excellent composition, but for me it seemed to be not economical, it was very liquid, and there was not yet a comfortable pump that sprayed the product all over the kitchen. Although there were no complaints about the detergent properties, but I was again in search of my ideal dishwashing liquid. And then I decided to look at the ecoville website again
And I found an agent for handwashing BIO Almawin



Economical IVF concentrate . Does not cause contact allergies and skin irritations. The ware dries well, does not require wiping. Wheat proteins and glycerin protect the skin of the hands. The product is completely biodegradable.
Application: Pour water into the sink, add 1 drop (2 ml) of the product. If heavily soiled, increase concentration.
Composition:
Sugar surfactant, organic sulphates from palm oil, ethanol, sodium chloride, benzyl alcohol, wheat proteins, glycerin, lactic acid, essential oils of lemon grass *, eucalyptus *, water (* certified organic). Moderate foaming.
Push I can say that with this tool I'm still satisfied, copes with its purpose perfectly, lasts a month, not as liquid as the previous one. The smell is excellent not obsessive, it smells like lemon, not chemical. It is also pleasant that it is colorless. Of course, ideally, it would be desirable without Ethanol and benzyl alcohol. I will look for something I can find without these components. If someone is familiar with dishwashing detergents with a more beautiful composition, so that there is no alcohol or alcohol at all, throw links, I will be grateful.
Thanks to those who read this article. Share your opinions. All happiness!
What really washes ?!
Surface active substances (surfactants) are, as a rule, chemicals that are contained in any cleaning agent, even in regular soap. Just thanks to the surfactant cleaning agent cleans. What are surfactants for?
Dirt, especially grease, is very difficult to wash off with water. Water will drain without washing off the fat. Water molecules do not stick to fat molecules and do not take them with them. Therefore, the task is to attach fat molecules to water molecules. This is exactly what surfactants do. The surfactant molecule is a sphere, one pole of which is lipophilic (it combines with fats), and the other is hydrophilic (it comes into contact with water molecules). That is, one end of the surfactant particle is attached to the fat particle, and the other end to water particles.
How do surfactants affect our skin?
However, most of the moisture in the human body also has a fatty basis. Those. for example, the protective layer of the skin (lipids - fats that protect the skin from various bacteria in the body) is a fatty film and is naturally destroyed by surfactants. And the infection attacks the place that is least protected, which of course is harmful to human health. Surfactants also destroy the body's cells (the activity of destruction depends on the type of surfactant). Experts say that after applying the detergent, the protective layer of the skin should have time to recover within 4 hours to at least 60%. These are the standards of hygiene established by state standard specification. However, not all detergents provide such a recoverable skin. Fat-free and dehydrated skin ages faster. In addition, surfactants can accumulate in the brain, liver, heart, body fat (especially a lot) and continue the destruction of the body for a long time. And since practically no one does without detergents, surfactants are constantly replenished in our body, providing continuous harm to the body. Surfactants also affect reproductive function in men, similar to radioactive radiation. The problem is aggravated by the fact that our wastewater treatment plants do not cope well with the removal of surfactants. Therefore, harmful surfactants are returned through the water supply system to us almost in the same concentration in which we pour them into the drain. The only exceptions are products with biodegradable surfactants.
Types of surfactants, features and which of them are biodegradable
According to the chemical nature of the hydrophilic group (soluble in water), surfactants are divided into four large classes: Anionic surfactants - in water solution decompose with the formation of negatively charged ions. The main advantage is the relatively low cost, efficiency and good solubility. But they are the most aggressive towards the human body. Cationic surfactants - in aqueous solution, decompose to form positively charged ions, possess a bactericidal property. Among cationic surfactants, quaternary ammonium compounds (QACs), imidazalines, fatty amines have the greatest value. Non-ionic surfactants - do not form ions in aqueous solution. The main advantage is a favorable effect on the fabric and most importantly - 100% biodegradability. Amphoteric surfactants - in aqueous solution, depending on the pH of the medium, can be cationic (in an acidic environment, pH<7) или анионные (в щелочной среде рН>7) properties. Some of the anionic (anionic) surfactants, for example, alkyl sulfonates, possess good biodegradability (by 80-98%). But nonionic surfactants have the most complete (100%) biodegradability. The inclusion of non-ionic surfactants in the formulation of detergents leads to a lower content of anionic substances on the skin. A similar effect, namely, a decrease in the accumulation of anionic surfactants on the skin and tissues, was established with the introduction of enzymes of biological origin in detergent compositions. One of the main criteria for the environmental safety of household and professional chemicals is the biodegradability of surfactants that are included in their composition. The company "Viyusa Bel" uses exclusively anionic and non-ionic surfactants. There are primary biodegradability, which implies structural changes (transformation) of surfactants by microorganisms, leading to the loss of surface-active properties. By complete biodegradability are meant the final biodegradation of surfactant to carbon dioxide and water.
The main use of surfactants is as an active component of detergents and cleaners (including those used for decontamination), soap, for the care of premises, equipment, tools, dishes, clothing, things, cars, etc. Currently, the most common surfactant in synthetic detergent is an alkyl benzene sulfonate. The group of anionic surfactants also includes alkanesulfonate (SAS), alkyl sulfate (FAS) and volatile alkyl sulfate (FAES). FAS can be obtained from vegetable raw materials, such as rapeseed oil, or coconut oil. In cationic surfactants, the hydrophilic group is represented by a positively charged, nitrogen-containing group. The negatively charged counterweight is chlorine ion, or methyl sulfate.
Why do you need a co-surfactant?
Since the actual detergent action is formed mainly by anionic surfactants, they form the basis of any detergent. The most common in the production of liquid soap is ethoxylated sodium laureth sulfate. A softer, but expensive substance - ethoxylated magnesium laureth sulphate - is usually used in baby cosmetics and products for sensitive skin. Oxyethylated ammonium laureth sulfate is often used in detergents manufactured in the USA. In European products, it is less common, since in these countries its dermatological properties are considered not high enough. Unfortunately, in the domestic market there are still widely distributed products containing only sodium laureth sulfate as a washing substance. These are very tough products, and whatever useful additives (most often plant extracts) are included in their recipe, they do not correct the situation. For the best removal of dirt, dissolving excess sebum, restoring the protective lipid layer with dermatological softness and good foaming, you must use a mixture of surfactants. Therefore, in addition to the main surfactant, Co-surfactant is always added to the detergent formulation. This is what helps to create an optimal balanced composition. As Co-surfactants, various amphoteric, non-ionic, anionic, and cryptoanionic surfactants are used. The ratio of surfactant and Co-surfactant is usually 1: 3, 1: 4. In practice, developers have to work out such ratios, taking into account the required cosmetic and technical characteristics. Sometimes with a combination of absolutely safe surfactants, the mixture exhibits undesirable dermatological effects. High demands on the purity of the environment induce the creation of biodegradable surfactants (biosurfactants). The fact is that synthetic detergents are practically not subject to natural decomposition processes, therefore they are able to accumulate in the environment, causing considerable damage to nature. Biodegradable surfactants are metabolic products of bacteria or components of membranes, that is, substances that in the external environment break down into the original non-toxic constituents. An attempt to create biosurfactants was carried out abroad. They are obtained biotechnologically in the process of growing various microorganisms: bacilli, fungi, pseudomonads. On a chemical structure, they are very diverse, but have a common advantage - they are safe from an environmental point of view.
On packs with detergents THERE ARE THESE THREE SAFETY SIGNS: "The sign of the cycle in nature." Safe packaging materials are used. Packaging can be recycled and reused. In addition, in nature, it decomposes without forming hazardous chemical compounds, such as dioxin. Thus, the purity of the soil and water is maintained. “Apple” - “Safety conscious formula”. Used composition is safe for our health and the environment. This mark is placed on the packaging of creams, personal care products, shampoos, etc. The hairsprays on which this sign stands do not use gases such as propane and butane. Varnishes are sprayed by mechanical action, and not by the pressure that propane, butane or other gases create in a hermetically sealed cartridge. When spraying varnish, we do not inhale these gases, and they do not enter the environment. “Bunny” - “No animal testing”. Not tested on animals. This is not necessary, the manufacturer uses only safe components. And thus no harm to animals. Typically, an animal test is needed in order to select the concentration of chemical components that will not lead to dangerous consequences for the animal, and therefore for humans.
"The sign of the cycle in nature." Safe packaging materials are used. Packaging can be recycled and reused. In addition, in nature, it decomposes without forming hazardous chemical compounds, such as dioxin. Thus, the purity of the soil and water is maintained. “Apple” - “Safety conscious formula”. Used composition is safe for our health and the environment. This mark is placed on the packaging of creams, personal care products, shampoos, etc. The hairsprays on which this sign stands do not use gases such as propane and butane. Varnishes are sprayed by mechanical action, and not by the pressure that propane, butane or other gases create in a hermetically sealed cartridge. When spraying varnish, we do not inhale these gases, and they do not enter the environment. “Bunny” - “No animal testing”. Not tested on animals. This is not necessary, the manufacturer uses only safe components. And thus no harm to animals. Typically, an animal test is needed in order to select the concentration of chemical components that will not lead to dangerous consequences for the animal, and therefore for humans.

