Amphoteric pav harm. Cationic pav
Most cationic surfactants contain a nitrogen atom that carries a positive charge, that is, they are amines or quaternary ammonium compounds. Amines exhibit surfactant properties only in the protonated state; therefore, they cannot be used at high pH. In contrast, quaternary ammonium compounds are insensitive to changes in pH. Amines are also more sensitive to the action of multiply charged anions. As already mentioned, ethoxylated amines have the properties of non-ionic and cationic surfactants, and the longer the oxyethylene chain, the more the non-ionic surfactants are expressed in such compounds.
In fig. 5. Some typical cationic surfactants are given. Quaternary ammonium compounds with ester groups represent a new class of environmentally friendly surfactants, displacing the corresponding di-alkyl derivatives in the process of softening of tissues.
Fig. 5. Structures of some typical cationic surfactants
Synthesis of non-ether quaternary ammonium surfactants passes through the formation of nitrile compounds. The fatty acid reacts with ammonia at elevated temperature to give the corresponding nitrile. This reaction goes through a step of forming an amide intermediate. Nitrile is then hydrogenated to the primary amine in the presence of a catalyst:
Secondary amines are obtained either directly from the nitrile, or in two stages from the primary amine. In a one-step process, which seems to go through the formation of an intermediate imine, ammonia is constantly removed from the reaction mixture to facilitate the formation of a secondary amine:
Primary amines are converted to long-chain 1,3-diamines by cyanoethylation:
Primary or secondary long chain amines can be methylated and converted to tertiary amines, for example, by reaction with formaldehyde under reducing conditions:
Ethylene oxide can also be used as an alkylating agent to convert primary and secondary amines to tertiary amines of the type R-CH2N2 and 2NCH 2 CH 2 OH.
Quaternary ammonium compounds are usually obtained from tertiary amines by reaction with a suitable alkylating agent, for example with methyl chloride or methyl bromide or dimethyl sulfate, and the choice of reagent is determined by the counterion surfactant:
Quaternary ammonium surfactants containing ester groups are obtained by esterification of the fatty acid with an amino alcohol followed by N-alkylation, as described above. As an example, the reaction of triethanolamine, taken as the amino alcohol, and dimethyl sulfate as the methylating agent is given:
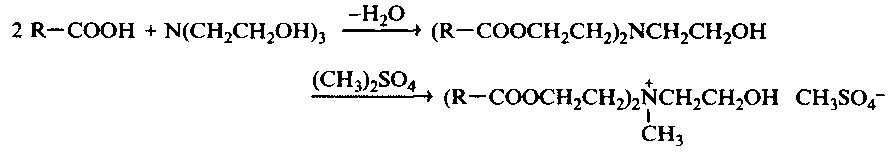
Sulfoxonium surfactants are obtained by oxidation of a sulfonate salt with hydrogen peroxide. The industrial use of non-nitrogen cationic surfactants is small, since these substances rarely have advantages over cheaper nitrogen-containing surfactants. Phosphonium surfactants with one sufficiently long alkyl chain and three methyl groups have been used as biocides.
Most surfaces — metals, minerals, plastics, fibers, cell membranes, etc. — are negatively charged. The main use of cationic surfactants is associated with their ability to adsorb on negatively charged surfaces. Some examples are given in table. 3, and the most important characteristics of cationic surfactants - in Table. 2
Table 2. The main characteristics of cationic surfactants
Table 3. The use of cationic surfactants due to their adsorption on surfaces
|
Surface |
Application |
|
Anticorrosion agents |
|
|
Minerals |
Flotation gatherers |
|
Inorganic pigments |
Dispersers |
|
Plastics |
Antistatic agents |
|
Antistatic agents, softeners |
|
|
Air conditioners |
|
|
Fertilizers |
To reduce caking |
|
Bacterial cell walls |
|


