Chemical methods for water quality analysis. Water analysis
How to determine the quality of natural water? For this you need to analyze natural water. Varieties of methods for determining the composition of water, their features. Express methods for studying natural water. Alternative testing techniques. Instruments for quick analysis in different conditions.
Not sure how to determine the quality of natural water? The main indicators of its quality are hardness, transparency, alkalinity and oxidation. But for this you need to analyze natural water.
Varieties of tests
Today, an analysis system operates in Russia, which is based on studying the microbiological and chemical characteristics of a liquid and further comparing the data obtained with the standard values.
The first type of measures allows you to identify the hardness of water, the presence of dry contents, as well as to find the amount of other substances of natural origin and elements caught in the liquid during the water treatment procedures.
The following three methods allow to find in the water even the minimum amount of carcinogenic and mutagenic contents (mercury, pesticides, antimony, aromatic carbohydrates, cyanides, various volatile mixtures, etc.).
Radiation control of natural waters allows to determine the total activity of the elements, as well as, if required, to identify the radionuclide composition of harmful impurities.
As a rule, water analysis is performed in several stages:
- The shortened stage of fluid analysis.
- Comprehensive chemical analysis.
- Conducting fluid studies for selected groups of indicators.
Usually, to determine the quality of natural water, rather abbreviated analysis. However, sometimes it is necessary to perform a comprehensive chemical analysis or conduct testing of hotel indicators.
Natural Water Analysis: Express Techniques
In addition to the organoleptic characteristics of water (odor, taste) with the help of hardware it is possible to carry out water monitoring of the composition of water. You can also perform express testing.
Today, for these purposes the following methods of analysis of natural water can be applied:
- Potentiometry
- Titrometry
- Turbidimetry
- Spectrophotometry
- Conductometry
- Nephelometry
- Flame photometry and conventional
- Fluorometry
- Gas chromatography
Using these techniques allows you to determine:
- Physical characteristics of water. Its acidity and hardness.
- The chemical composition, that is, the number of elements of iron, nitrates, chlorine, the presence of particles of heavy metals. At this stage, you can determine the permanganate oxidation of water.
- The toxicological composition of the liquid, namely the PKD indicator.
Of course, the fastest water analysis can be done by each of us independently. For example, having tasted the water from our water supply systems, you will definitely feel the presence of chlorine, after drinking country water, you can taste with confidence that there is iron in the composition. And if water is defended for a long time, then a white precipitate forms at the bottom of the container, which speaks of salt impurities. However, such testing methods are very subjective, so there is a risk of making a mistake. In order to accurately calculate whether you can drink water or not, you need to analyze the drinking and natural waters.

Alternative Water Testing Techniques
The analysis of natural and waste waters can be performed using bacteriological, chemical-physical and biological methods of quality assessment. Each of these methods has its pros and cons.
- The physico-chemical method allows one to study the chemical and physical characteristics of the fluid at the desired time interval, as well as to track the interaction of these indicators between themselves. The advantage of the method is the high accuracy of the results with minimal error. Disadvantage: the method allows you to explore only the abiotic indicators of the liquid, which does not give a complete picture.
- Bacteriological methods detect water quality based on the presence of pathogenic microorganisms. Pluses methods: high accuracy, the possibility of wide application. Disadvantages: the technique can be used only in a sterile laboratory environment. Selected samples of water must be stored under certain conditions. For the analysis, you need a specialist bacteriologist and laboratory technician.
- Biological methods provide an opportunity to explore the indicators, which at the first stage cannot be identified. This method helps to determine the sanitary state of the fluid, the level and type of pollution, the degree of its distribution in the reservoir. Also with the help of this method it is possible to characterize the course of self-purification processes. Cons: it is required to take a set of samples in different places. All this will take a lot of time. You will need to attract a specialist hydrobiologist. Restrictions of the season. It is impossible to track the rapid change in the level of pollution of the reservoir.
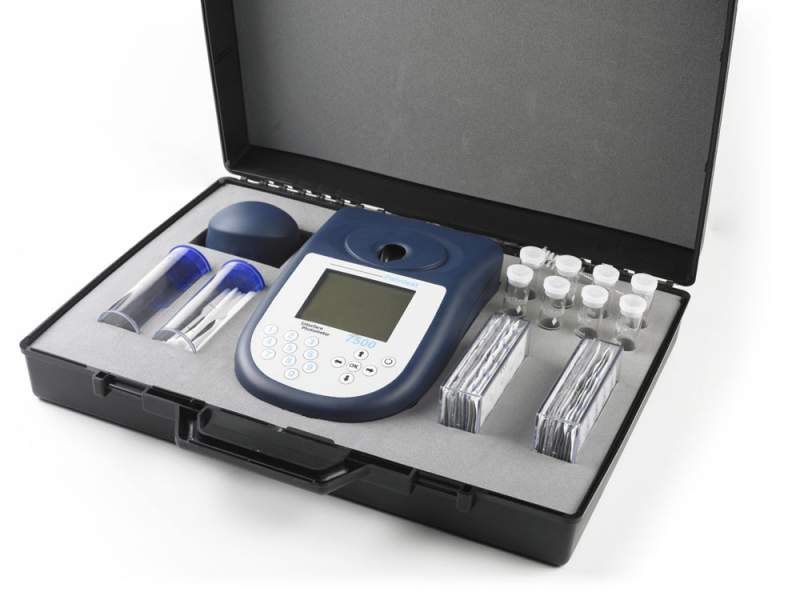
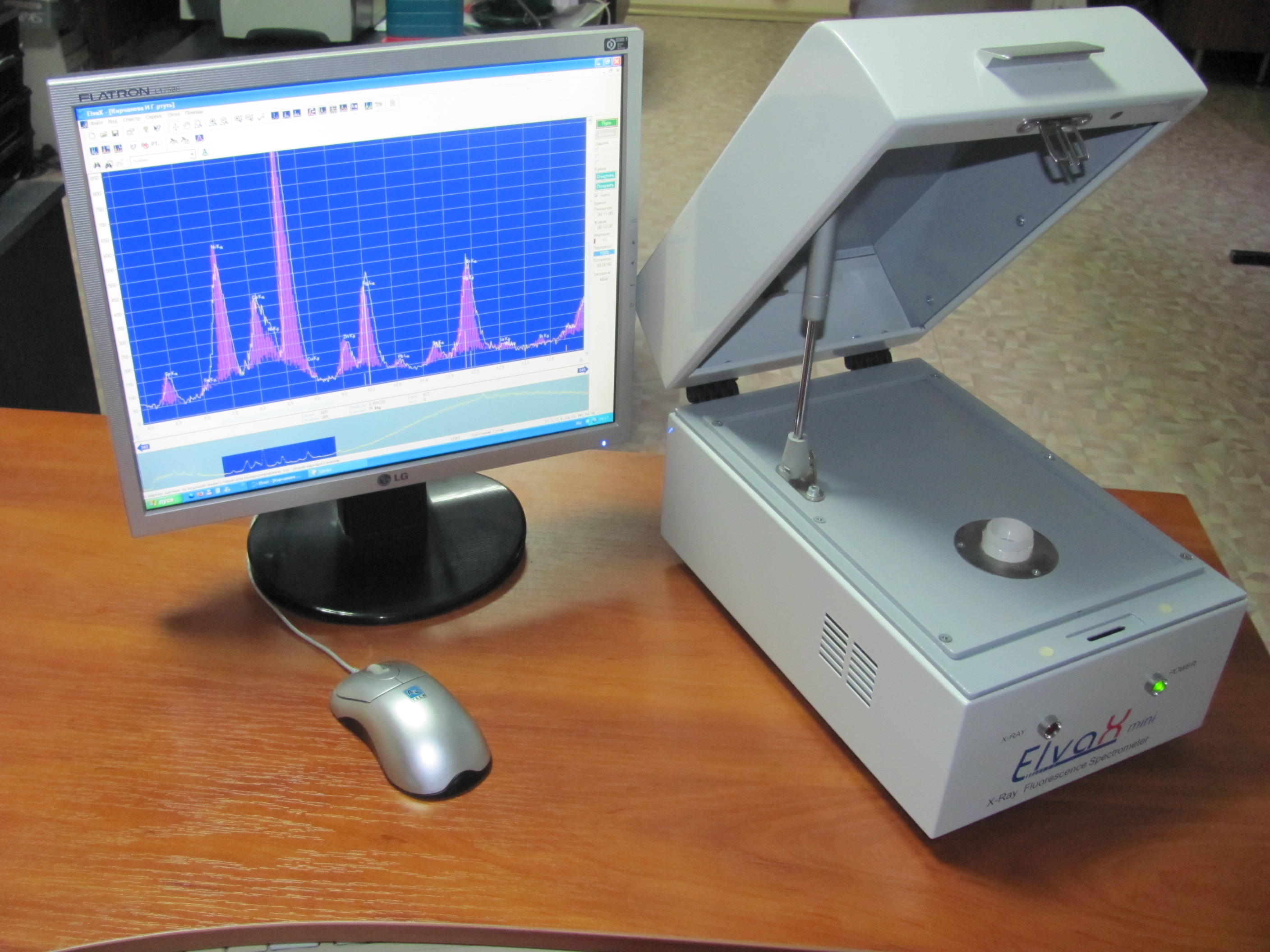
Instruments for the analysis of natural water
For the chemical analysis of natural and waste water, you can use a variety of portable devices that are suitable for use in different conditions. Typically, such devices come with the required reagents, devices (compact photocolorimeters, spectrophotometers) and indicators. For example, devices CHEMetrics.
This unit has everything you need to conduct thirty types of fluid analysis. The accuracy of the device is quite high. It has self-filling capsules for water samples. The duration of the analysis is five minutes.
The device allows to determine 5 main indicators of water quality:
- Chemical characteristics.
- Organoleptic.
- Toxicological.
- Microbiological.
- Are common.
Want to order a water test? You call, by phones specified on the website, our experts will carry out water intake and all necessary analyzes.
High-quality water analysis is mandatory carried out before putting the source into operation. Water must meet the requirements of SanPiN 2.1.4.1175-02, comply with the standards for microbiological, organoleptic, chemical indicators. In addition to the initial study, it is recommended to periodically analyze the quality of water in an already operated source to find out if there is any contamination that is indefinable by eye, and to take measures if it has occurred (the analysis will not only show the composition of the liquid, but also allow to establish the causes of its changes).
According to SanPiN 2.1.4.1175-02, water must meet 16 standards: making the usual qualitative analysis, it is these 16 parameters that are checked. It should be understood that the deviation of at least one of the permissible values makes the water unsuitable for consumption. There is still an advanced analysis of natural water (check 25 indicators), it is optional, but relatives who value their health and health prefer a broad study, since the wells are at risk because of the small depth.
Parameters are divided into three groups:
- Microbiological.
- Organoleptic.
- Chemical.
These are three separate studies conducted during the water quality analysis process.
Microbiological parameters of natural water
Microbiological parameters - the presence / absence of coliform bacteria in water and microbes forming colonies. Coliphages, plaque-forming units, common and thermotolerant coliform bacteria should be absent (hepatitis A, dysentery and other diseases often occur due to bad water). Permissible presence of microbes is negligible - 100 / ml.
The result of microbiological analysis
Organoleptic parameters of natural water
The organoleptic parameters of water are its qualities perceived by the senses (taste, sight, touch, hearing): smell, turbidity, taste, color.
If the water has become red, there is a surplus of iron in it (as an option), and this can be seen with the naked eye. However, it is better not to bring such a state up: a slight color may not be visible to the eye, but the body will receive an increased dose of iron, moreover, already oxidized. The same goes for turbidity. Smell and taste - more defined parameters.
Smell
Water is given a smell of various liquid and organic substances of natural and artificial origin. Marsh, putrid, sulfuric - natural "flavors". The odor source is a waste product of anaerobic bacteria that live in the clay sediment at the bottom of the well. The smell of artificial origin create oil, phenolic, chlorine and other impurities. The parameter is estimated on a five-point scale. Valid value is a maximum of 3.
Turbidity
This parameter depends on the presence and amount of fine suspensions. Turbidity is not always possible to determine visually (it can be seen when the content of insoluble particles in the water is off scale), but when analyzing natural water, it is evaluated by the dry residue after filtration. Another method for determining turbidity is photometry (assess the quality of a light beam passing through water).
The cause of turbidity is an increase in the concentration of various impurities: this is mainly clay and silt. For well water is characterized by a seasonal increase in turbidity, when the thawed and storm waters wash away the soil. Less commonly, the water becomes cloudy due to increased levels of iron and humus.

When conducting a qualitative analysis, it is necessary to estimate the color and turbidity of the water.
Smack
Bitter, sour, sweet, salty - this is the taste. Everything else - tastes: ammonia, metal, chlorine and others. They are determined by the presence of impurities (they do not taste to the taste), including when heated (at high temperatures, an increase in the effect is observed). In the assessment using a five-point scale (maximum 3 points is permissible). The main reason for exceeding the permissible values - industrial pollution.
Colour
Water takes on different shades depending on the substances in it. The appearance of a brownish or yellow tint indicates an increased iron content. Peat deposits also stain water yellow. Clay gives a reddish tint. None of these impurities is safe, and there are many others. To identify the cause of the color change and take appropriate measures (to choose the right cleaning filters), you need to make a qualitative analysis of natural water.
Chemical parameters of natural water
Chemical parameters show not only the content of substances of artificial origin, but also of natural (calcium, magnesium). Qualitative analysis of natural water determines the content of elements, the concentration of organic and inorganic substances. Chemical parameters include:
- Hydrogen indicator.
- Rigidity.
- Nitrate (NO3) content.
- Water salinity (total).
- Permanganate oxidability.
- Sulfate content (SO4 (2-)).
- Chloride content (CL (-)).
- Concentration of organic, inorganic chemicals.
Rigidity
Hard water make calcium and magnesium salts, which turn into insoluble under the influence of temperature. Water hardness is the cause of the formation of deposits in pipes and boilers, scum on the walls of household appliances (kettle, washing machine, dishwasher, etc.). Calcium and magnesium affect the work of the cardiovascular and urogenital systems of the human body, therefore their content in water is strictly standardized.
Dry sediment consists of organic elements; inorganic salts dissolved in water. Upon contact with oxygen, soluble compounds are oxidized and precipitate, taking an insoluble form. The main reason for the sediment is iron, manganese (their high content). In the process of qualitative water analysis, the composition of the precipitate and the quantitative indicator are determined: it should not exceed 1500 mg / l.
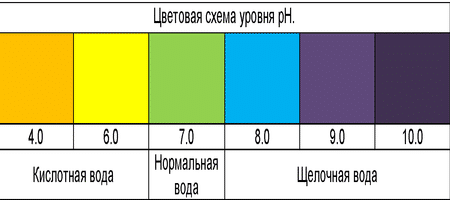
Hydrogen indicator
The pH is measured in pH units (normally: from 6 to 9). Deviations indicate the excess / insufficiency of the permissible content of alkalis and acids in water. With an insufficient pH level, the water is acidic, with an elevated level - alkaline.
How to make a qualitative analysis of water
High-quality water analysis is carried out in special laboratories. The accuracy of the analysis depends not only on the reagents, strict adherence to the procedure and laboratory equipment, but also on competent sampling. Observe the following rules:
- capacity for water intake must be sterilized;
- capacity - at least 1 l;
- do not use bottles of sweet sparkling water (this will distort the results);
- for microbiological analysis, it is necessary to take a sample as quickly as possible so that bacteria do not spoil the sample from the air;
- selected water must be delivered to the laboratory within 24 hours.
Just three steps to get a qualitative analysis.
It is not worth bringing to a situation when it is already possible to visually make a water analysis, having determined its unsuitability by eye. Even if we reject the legislation and all other clever theses, the bare facts will remain:
- Fluorine salts provoke the development of fluorosis and caries.
- Molybdenum increases the acid content in the blood, urine.
- Mercury affects the central nervous system.
- Neurotoxic aluminum, with its steady tendency to accumulate, enters the liver and brain, disrupting their function.
- Arsenic provokes the development of cancer.
Each element present in the body - barium, beryllium, iron, manganese, copper, etc. - turns into poison with an increased content in water. And these are only safe, in principle, elements, but there are also bacteria and industrial pollution.
The analysis can be done independently, but it will only determine the presence of impurities, their approximate quantitative characteristic, but not the qualitative component. For example, a certain amount of iron is not dangerous, but mercury in the same amount is poison. Even developers of tests showing the presence of metals in a liquid are recommended to give water for professional analysis.
Video: water analysis for heavy metals at home
The analysis can be done independently, but it will only determine the presence of impurities, their approximate quantitative characteristic, but not the qualitative component.
For example, a certain amount of iron is not dangerous, but mercury in the same amount is poison.
Even developers of tests showing the presence of metals in a liquid are recommended to give water for professional analysis.
When heated, the bicarbonate ion decomposes, so it is easy to get rid of temporary hardness by simple boiling. In the laboratory of analysis of the quality of drinking water, the content of bicarbonate ion is determined by the alkalinity index.
When gypsum is dissolved, sulfates enter natural waters. In drinking water, their concentration should not exceed 500 mg / l. At higher concentrations, gastrointestinal disturbances are possible. The same concentration contributes to the corrosion of concrete and metal. Sulfates can also get into natural waters from atmospheric emissions from motor vehicles and industry. Analysis of water for sulphate content implies a suspension of low soluble barium sulphate and spectrophotometry of this suspension.
The concentration of chlorides in fresh water is not high. An increase in the content of chlorides in drinking water may indicate its contamination by wastewater from enterprises in which they are contained in large quantities. The maximum permissible concentration of chlorides in drinking water is 300 mg / l. The chloride content above this threshold makes the water taste salty. The analysis of drinking water in the quality control laboratory is carried out by titrating the sample with silver nitrate solution.
In addition to the main ions, natural waters contain dissolved gases. The most important of these is oxygen. Its content in natural waters depends on the season, the depth of the reservoir, the conditions of aeration, the activity of microorganisms, plants and animals. The decrease in the oxygen content in natural water is the result of organic compounds in the pond. The maximum oxygen content in natural waters is observed in summer due to the active photosynthesis of plants. Dissolved oxygen gives the water a refreshing taste. The analysis of natural water for the content of dissolved oxygen is complicated by the fact that its chemical fixation is necessary immediately after sampling.
Carbon dioxide, dissolving in water, interacts with it to form carbonic acid. Separately analytically determine the content of carbon dioxide and carbonic acid in water is impossible, therefore, the total concentration of these components is taken as the concentration of free carbonic acid.
When carbonic acid dissolves sulfide minerals, and also as a result of the biochemical decomposition of sulfur-containing organic compounds, hydrogen sulfide is formed in water in the absence of oxygen. Hydrogen sulfide is a toxic compound. It gives the water an unpleasant characteristic smell of "rotten eggs." In drinking water, the content of hydrogen sulfide is not allowed. The analysis of drinking water in the quality control laboratory is performed using an ion-selective electrode.
In addition to inorganic compounds, a large amount of organic substances that form the so-called “aqueous humus” are dissolved in natural water. Organic matter enters water bodies due to their flushing from the surface of the soil. Due to the increase in anthropogenic pressure on the environment, municipal wastewater and wastewater from enterprises have become a source of organic matter in natural water. The content of aqueous humus can reach 50 mg / l and more. The largest part of it consists of fulvic acids, which provide the characteristic brown-brown or yellowish color of the natural waters of most rivers. Water analysis for the content of organic compounds produced by permanganate oxidation. At the same time, the value of permanganate oxidizability up to 5 mg O2 / l is considered acceptable. Permanent oxidation above 5 mg O2 / l indicates water pollution with organic matter effluent.
Any water contains the so-called "biogenic" elements, i.e. those elements that make up most of living organisms. These include primarily nitrogen and phosphorus.
Nitrogen in natural waters is contained in the organic form (proteins, amino acids) and inorganic - ammonium, nitrite and nitrate. Ammonium and nitrates are formed in natural water as a result of the decomposition of proteins. In addition, nitrates can get into the water when nitrogen fertilizers are washed away from the fields and when nitrogen oxides are dissolved in rainwater. Once in the human or animal body, nitrates are restored by the intestinal microbiota to nitrites, which interact with the respiratory blood protein, hemoglobin. In this case, hemoglobin is restored to methemoglobin, which is not able to carry oxygen.
The maximum permissible nitrate content in drinking water is 45 mg / l, nitrite - up to 5 mg / l, ammonium - up to 2.5 mg / l. The analysis of water in the laboratory for the content of nitrates and nitrites is based on obtaining colored solutions of these forms of nitrogen with Griess reagents. The analysis of ammonium water is based on the preparation of a colored ammonium compound with mercury salts (Nessler's reagent).
Iron is contained mainly in groundwater, because in the surface waters, complete hydrolysis of its ions occurs. When the concentration of iron ions is higher than 0.3 mg / l, water acquires a ferrous taste. Iron ions along with calcium and magnesium salts provide water hardness. Water analysis for iron is performed on an atomic absorption spectrophotometer.
The elements contained in water in quantities less than 1 mg / l are called trace elements. As a rule, these are extremely important elements for the normal functioning of the body. Among them should be mentioned iodine and fluorine. Iodine compounds in natural waters are contained in the form of iodide ions. Its concentration is in thousandths of mg / l. With insufficient iodine in drinking water, diseases of the thyroid gland and of the brain develop. When the content of iodine is from 0.0001 to 0.001 mg / l, the diseases are observed on average in 1.5-3% of people, and in the content of iodine from 0.0014 to 0.01 mg / l - only in 0.1%.
Fluorine in natural waters is contained in the form of fluoride ions. Its content can reach 10 mg / l. Fluorine is actively involved in the process of mineralization of human bone tissue. With insufficient fluorine content in water, the development of caries occurs, and with excessive enamel spotting, fluorosis occurs.
Water analysis for the content of iodide ions and fluoride ions is carried out in the laboratory monitoring the quality of drinking water using ion-selective electrodes.
The source of water supply is an artesian well, the Republic of Karelia, the village of Vyatkya (sample 1).
Water after filtration - (information on filtering loading is absent, sample 2).
Rated indicators - according to hygienic requirements for clean drinking
water systems water SanPin 2.1.4.1074-01.
Summary table of water quality indicators:
Table 1
|
Name indicators |
initial |
after filtering |
SanPin 2.1.4.1074-01 |
|
|
Chromaticity, hail |
||||
|
Turbidity, mg / l |
||||
|
Scent, score |
||||
|
Hardness, 0 F |
||||
|
Ammonia nitrogen, mg / l |
||||
|
Nitrites, mg / l |
||||
|
Nitrates, mg / l |
||||
|
Oxidizability, MCO 2 / l |
||||
|
Chlorides, mg / l |
||||
|
Sulfates, mg / l |
||||
|
Fluorine, mg / l |
||||
|
Iron mg / l |
||||
|
Manganese, mg / l |
||||
|
Zinc, mg / l |
||||
|
Copper mg / l |
||||
|
Lead mg / l |
||||
|
Cadmium mg / l |
A brief description of the indicators and their effects on the human body.
1. pH - concentration of hydrogen ions in solution.
The determination is carried out in the range from 10 to 10-14 mol / l, which corresponds to
pH value from -1 to 14.
The neutral state of the solution at a temperature of 220 ° C corresponds to pH = 7,
the decrease in the value is acidic, and the increase is alkaline.
Sample 1 - pH = 6.9 - neutral medium.
Sample 2 - pH = 8.0 - alkaline medium, the increase is tied to the filter material.
Over time, after passing a certain amount of water, a decrease is possible.
Recommended: pH = 6-7.
2. Chroma - water quality indicator, which characterizes the intensity of water color.
The number of substances affecting the color depends on the geological conditions of the aquifers, the nature of the soil, etc. The smallest color is observed in groundwater. The decrease depends on further water purification.
3. Turbidity - caused by the presence of fine suspended organic and
inorganic origin. Increased turbidity can be caused
release of carbonates, hardness salts (see section 5), oxidation of iron compounds and
manganese (p.13,14).
Turbidity adversely affects the appearance of water and protects microorganisms during
ultraviolet disinfection and stimulates the growth of bacteria.
The reduction can be achieved by reducing the stiffness, iron oxides and manganese.
4. Smell - refers to the organoleptic characteristics of water. The smell is due to the properties-
mi raw water and its processing methods. The smell of water is characterized by the kinds of smell and
intensity. The smell of water is influenced by the composition of solutes
temperature, pH value. The intensity of the smell of water is determined experimentally.
at 200C and 600C and measured in points, according to the requirements.
5. Stiffness - water hardness is called the property of water due to the presence in
it dissolved calcium and magnesium salts.
Total stiffness is the sum of carbonate (temporary) and non-carbonate (constant) stiffness. Carbonate hardness due to the presence of water
bicarbonates and non-carbonates (at pH\u003e 8.3) calcium and magnesium. When heated, bicarbonates decompose to form carbonic acid and precipitate.
calcium carbonate and magnesium hydroxide. This type of stiffness is almost eliminated by
boiling and therefore called temporary. Non-carbonate stiffness due to
the presence of calcium and magnesium salts of strong acids and boiling is not
eliminated (constant stiffness).
In test 1, 2, the total stiffness was determined. According to the classification of hardness, the test water belongs to the type of soft waters (up to 3 megaq / l).
The taste threshold for calcium ion is in the range of 2-6mgqv / l, the taste threshold for magnesium is lower. World Organization
Health does not offer any recommended stiffness value
indications of health effects. The WHO materials state that although a number of studies revealed a statistically inverse relationship between the hardness of drinking water.
and cardiovascular diseases, the available data are not enough to derive
about the causal nature of this connection. Definitely not proven that soft water has
negative effect on the balance of minerals in the human body.
6. Nitrogen ammonia - ammonium nitrogen (NH3 and NH4). According to WHO, the norm is 1.5 mg / l.
This indicator is judged on the contamination of the water source, as an example:
7. Nitrites - salts of nitrous acid. Nitrite anions are intermediate
products of the biological decomposition of nitrogen-containing organic
compounds. Due to the ability to turn into nitrates, nitrites are usually
absent in surface waters. MPC of nitrite (for NO2) in the water reservoirs
is 3.3 mg / l. An increased amount of nitrogen in natural water indicates
on pollution of the source by sewage. Therefore, in drinking water in general is not allowed the presence of organic and ammonium nitrogen.
8. Nitrates - salts of nitric acid. Opportunities for drinking nitrate pollution
the waters depend on the depth, well design, characteristics of the groundwater formation, as well as climatic conditions. In many cases, the time required for
the flow of nitrate through the soil into groundwater is difficult to predict.
Increased doses of water and food intake, reduce the ability of blood
to oxygen transport.
9. Permanganate oxidation - allows you to assess the presence of water only easily
soluble substances such as sulfides, nitrites, ferrous iron, some
humic substances. It is expressed in the number of milligrams of oxygen, necessary
for oxidation under certain conditions of organic substances contained in 1l
water. For groundwater, this is a small amount.
10. Chlorides - are present in almost all fresh surface and groundwater.
waters, and also in drinking water in the form of metal salts. Mostly their presence in
water is associated with leaching of sodium chloride from rocks (sodium chloride).
It is on the organoleptic index - taste and approved by the MPC of drinking water.
on chlorides (350 mg / l).
Filter material, its sorption properties, allowed to reduce the content
chlorides (sample 2) to 3.8 mg / l.
11.Sulfates - natural sulphate content in surface and groundwater
due to weathering of rocks and biochemical processes occurring
in aquifers of the earth. Deterioration of water taste and sulphate taste
occurs when their concentration is 250-400 mg / l.
An elevated sulfate content in water leads to gastrointestinal upset. Sulfate water purification is carried out comprehensively and is aimed at
decrease in total salt content of water.
12. Fluorine - (fluorides). Fluoride in the form of fluoride can be contained in natural and groundwater. The concentration of fluoride in drinking water should be no more than 1.5 and no less than
0.5 mg / l., Therefore, with an excess of fluorine in natural water, it is defluted;
disadvantage - fluoridate. Excess fluoride in the body causes the destruction of dental
enamel, precipitates calcium, which leads to a violation of calcium and phosphorus
exchange. For these reasons, the determination of fluoride in drinking water as well as waters
artesian wells is very important.
The limiting indicator of harm - sanitary and toxic.
13. Iron - common. Almost always present in groundwater, concentration
depends on the geological structure and hydrogeological conditions of the basin. Iron compounds in water are present in dissolved, colloidal and undissolved form.
The high iron content worsens the taste of drinking water. According to the evidence of harm
for health, this figure is not installed. In the presence of an oxidizing agent, bivalent iron is oxidized and turns into a trivalent form, forming a poorly soluble
iron hydroxide precipitating. Thus, to remove iron oxides
for, you need a strong oxidizing agent and a pH of 8.5-9.0.
14. Manganese - in its pure form is not found in nature. In the ores it is present
in the form of oxides, hydroxides and carbonates. Always present in manganese ores
iron. It is not as common as iron, but is very similar to it.
its properties.
According to the World Health Organization (WHO) since 1998. are set
very strict norms of the maximum content of manganese in tap water -
0.05 mg / l. SanPin norms allowable manganese content in water supply
drinking water - 0.1 mg / l. Increased manganese content in water is unfavorable.
affects the higher nervous activity of man, there is a decrease
blood enzyme activity. Pay attention to reduce the amount of water.
15. Zinc - contains in the form of cations or in the form of complex anions. Zinc
may be present in water-insoluble forms - in the form of hydroxide,
carbonate, sulfide. The role of zinc in the body’s vital activity is to
that it is part of more than 40 important enzymes. Zinc affects taste and smell.
Water purification from zinc ions is complex, based on filtration processes and
sorption. The decrease in zinc ions after purification by 16-28%.
16. Copper - may be present in various forms: ionic, complex, colloidal
and in contact with air to change its state.
The copper content in drinking water should not exceed 2 mg / l over a period of 14 days.
However, copper deficiency in drinking water is also undesirable. Copper ions impart
water metallic taste. Different people have a taste threshold for copper
makes 2-10mg / l. The natural ability for such a definition is increased
the copper content in water is a natural defense mechanism of the body.
Bactericidal properties of copper The Federal Agency for Environmental Protection
United States (USEPA), officially assigned to copper and several copper alloys, the status of substances
with a bactericidal surface.
17. Lead - fine.
18. Cadmium - to a series of specific impurities can be attributed, the presence of which
affects the organoleptic, hygienic and chemical properties of water, as well as
has an impact (often negative) on the applied technological methods
purification of natural waters. To the number of toxic substances whose content in drinking
water is strictly limited; beryllium, molybdenum, arsenic, lead, selenium,
strontium, cadmium, mercury, cyanide, polycyclic carbons.
It should be noted that when several substances or substances are found in water
non-toxic, but affecting the taste of water, their total concentration, expressed
in shares of the MPC standards, should not be greater than 1. The calculation corresponding to these
requirements is carried out according to the formula:
c1 / C1 + c2 / C2 + ... + cn / SP = 1 (1)
where с1, с2, сП - concentrations found in water, mg / l; C1, C2, Cn - established
MAC, mg / l.
Threshold or maximum permissible concentration (MPC) of specified elements
small enough. If several elements of the same type of action are in
water at the same time, the concentration of each of them should be no higher than calculated by the formula (1).
An example of the calculation of the content of 2company in the sample2:
<0,01/0,01 + <0,001/0,01=1 в пределах допустимых концентраций.
Evaluation of sanitary - bacteriological indicators of water quality.
Summary table of indicators:
table 2
|
Name indicators |
initial |
clarified |
Normalized indicators |
|
|
Total microbial count |
no more than 100 |
|||
|
Common coliform bacteria |
not found in 100ml. |
not found. |
lack of |
|
|
Thermotolerant coliform bacteria |
not detected |
not found. |
lack of |
Brief description of indicators:
Water safety is epidemically determined by the number of microorganisms.
and the number of coliform bacteria. By microbiological indicators
drinking water must meet the requirements of SanPin 2.1.4.1074-01.
1. Total microbial count - the number of bacterial colonies formed in 1 ml.
* Rated indicator - no more than 50 CFU / ml.
2. Coliform organisms are convenient indicators of characteristics.
microbiological state of water quality.
3. Thermotolerant coliform bacteria - amenable to rapid detection and
play an important secondary role in assessing the effectiveness of water purification from fecal bacteria (E. coli).
Conclusion:
1. Comparative evaluation of the composition, properties of source water and purified water with standardized indicators, showed its suitability for drinking as a drinking water.
2. The epidemic is safe water.
1. To improve water quality and reduce the content:
- manganese;
- iron;
- nitrite;
- partially metal salts;
- chromaticity;
- hardness salts
to supplement the technological scheme of water treatment with another step - a softener filter.
This stage of water treatment will not only improve the quality of drinking water, but also
will minimize the cost of repairing equipment, prevent the occurrence
deposits of water heating equipment (hardness standards up to 30 µgq / l).
The analysis methods developed for surface fresh and salt waters are undoubtedly applicable to the analysis of other water bodies, including groundwater and lysimetric waters, soil solutions and extracts.
The analytical procedure for determining the content of elements in waters of different composition includes several stages:
Sampling;
Sample preparation;
Actually instrumental analysis.
Depending on the concentrations of the elements to be determined and the capabilities of the instrumental engineering, the above stages may be complicated by the introduction of additional steps related to the conservation of the analyzed samples, preliminary concentration of the elements and equipment modernization (for example, the introduction of additional devices for sample introduction, transfer from one aggregative state to another, and .d.)
Sampling and sample preparation as the most important stage of analysis. The sampling of water should be considered as a stage that largely determines the correctness of the subsequent analysis, and the errors made during the sampling process can no longer be corrected even by the most qualified analyst. The location and conditions for sampling water in each case are determined by specific research tasks, but the basic rules for sampling are of a general nature:
A water sample taken for analysis should reflect the conditions and place of sampling;
Sampling, storage and transportation should exclude the possibility of changing its initial composition (contents of the determined components or water properties);
The sample size should be sufficient to conduct an analytical procedure in accordance with the methodology.
Water sampling. Water sampling can be one-time and serial. Single selection is usually used to obtain initial information about the quality of the analyzed water. Taking into account the composition of the analyzed waters, which varies in time and space, serial selection is more justified, which is carried out either from different depths of the source, or at different points in time. With this selection, you can judge the change in water quality over time or depending on its flow.
In their mind, the samples are simple and mixed. Simple trial provided by a single selection of all required for the analysis of the amount of water, while the information obtained corresponds to the composition at a given point at a given time. Mixed sample obtained by pouring simple samples taken at different time intervals or at different points, thus characterizing the average composition of water. If a sample is taken from an open watercourse, it is necessary to observe the conditions under which it will be typical: the best places for sampling are turbulent areas where more complete mixing occurs. When sampling waste water, the following conditions must be met:
Sampling rate of at least 0.5 m / s;
The diameter of the sampling hole is not less than 9-12 mm;
High turbulence (in the absence of create artificially).
When sampling drinking water, it is necessary to first flush the water for 15 minutes with the valve fully open. Before closing the vessel with a stopper, the top layer of water is drained so that an air layer with a volume of 5-10 cm 3 remains under the stopper.
For sampling and storage of samples using dishes made of glass, polyethylene, Teflon. For the determination of ultramicroconcentrations of elements, an ideal material for sampling and especially for storage of samples is the new polymer polytetrafluoroalkoxy ethylene (PFA). Its main advantages compared with Teflon, used in analytical chemistry of trace elements, are high hydrophobicity and the almost complete absence of internal pores, and hence the absence of the "memory" effect.
Conservation and storage. A sampled natural water is a two-phase system consisting of a solution and a suspended substance. To avoid the loss of trace elements due to biochemical processes and sorption on the vessel walls, the sample after filtration is preserved, in some cases even unfiltered samples, if this is consistent with the research task.
For preserving metals, acidification of the sample with nitric acid to pH is usually used.< 2, причем во избежание загрязнения пробы микроэлементами во время консервации кислоту предварительно очищают суббойлерной перегонкой.
Samples of drinking water canned only if it is impossible to conduct an analysis on the day of selection.
It is difficult to preserve wastewater, since the introduction of a preservative may lead to side processes that complicate the analysis. In some cases, biochemical processes in wastewater samples can be slowed down by cooling and storage at 3 - 4 "C. The latter is also effective for samples of natural waters.
It should be noted, however, that despite the above recommendations on water sampling, the proportion of sampling error in the total error of analysis can reach 80% or more. Improving the accuracy of the analysis can be achieved by means of mobile analysis.


