Phosphoric anhydride
The element phosphorus forms a number of oxides, the most important being phosphorus(III) oxide. P2O3 and phosphorus(V) oxide P2O5 .
Phosphorus (III) oxide, or phosphorous anhydride (P2O3) obtained by the slow oxidation of phosphorus, burning it in a lack of oxygen. It is a waxy crystalline white mass with a melting point of 22.5 °C. Poisonous.
Chemical properties:
1) reacts with cold water, thus forming phosphorous acid H3PO3;
2) interacting with alkalis, forms salts - phosphites;
3) is a strong reducing agent.
Interacting with oxygen, it is oxidized to phosphorus (V) oxide P2O5.
Phosphorus oxide (V), or phosphoric anhydride (P2O5) obtained by burning phosphorus in air or oxygen. It is a white crystalline powder with a melting point of 36 °C.
Chemical properties:
1) interacting with water, forms ortho-phosphoric acid H3PO4;
2) having the properties of an acidic oxide, it reacts with basic oxides and hydroxides;
3) capable of absorbing water vapor.
Phosphoric acids.
Phosphoric anhydride corresponds to several acids. The main one is phosphoric acid H3PO4. Anhydrous phosphoric acid is presented in the form of colorless transparent crystals with a melting point of 42.35 ° C and readily soluble in water.
Forms three types of salts:
1) medium salts - orthophosphates;
2) acidic salts with one hydrogen atom;
3) acidic salts with two hydrogen atoms.
Getting phosphoric acid:
1) in the laboratory: 3P + 5HNO3 + 2H2O = 3H3PO4 + 5NO?;
2) in industry: a) thermal method; b) extraction method: Ca3(PO4)2 + 3H2SO4 = CaSO4? + 2 H3PO4.
Natural phosphates are reduced to free phosphorus, which is burned in air or oxygen. The reaction product is dissolved in water.
The remaining phosphoric acids, depending on the method of connection of the PO4 groups, form 2 types of acids: polyphosphoric acids, which consist of chains - PO3-O-PO3-... and metaphosphoric acids, which consist of rings formed by PO4.
Application: orthophosphoric acid is used in the production of fertilizers, chemical reagents, organic compounds, and for the preparation of protective coatings on metals. Phosphates are used in the production of enamels and pharmaceuticals. Metaphosphates are part of detergents.
– NH4H2PO4 or (NH4)2H2PO4.
Nitrophoska obtained by fusing ammonium hydrogen phosphate, ammonium nitrate and sodium chloride (sulphate).
38. Carbon and its properties
Carbon (C) is a typical non-metal; in the periodic system is in the 2nd period of the IV group, the main subgroup. Ordinal number 6, Ar = 12.011 amu, nuclear charge +6. Physical properties: carbon forms many allotropic modifications: diamond one of the hardest substances graphite, coal, soot .
Chemical properties: electronic configuration: 1s22 s22p2 . On the electron shell of an atom - 6 electrons; at the outer valence level - 4 electrons. The most characteristic oxidation states: +4, +2 - in inorganic compounds, -4, -2 - in organic ones. Carbon in any hybrid state is able to use all of its valence electrons and orbitals. 4-valent carbon has no unshared electron pairs and no free orbitals - carbon is chemically relatively stable. There are several types of hybridization: sp, sp2 , s p3. At low temperatures carbon is inert, but when heated, its activity increases. Carbon is a good reducing agent, but when combined with metals and forming carbides, it acts as an oxidizing agent:
Carbon (coke) reacts with metal oxides:
In this way metal is smelted from ore. At very high temperatures, carbon reacts with many non-metals. It forms a huge number of organic compounds with hydrogen - hydrocarbons. In the presence of nickel (Ni), carbon, reacting with hydrogen, forms a saturated hydrocarbon - methane: C + H2 = CH4.
When interacting with sulfur, it forms carbon disulfide: C + 2S2 = CS2.
At the temperature of an electric arc, carbon combines with nitrogen, forming a poisonous gas. dician: 2С + N2 = С2N2?.
In combination with hydrogen, cyanogen forms hydrocyanic acid - HCN. Depending on their chemical activity, carbon reacts with halogens to form halides. In the cold, it reacts with fluorine: C + 2F2 = CF2.
At 2000 °C in an electric furnace, carbon combines with silicon, forming carborundum: Si + C = SiC.
Finding in nature: free carbon occurs as diamond and graphite. In the form of compounds, carbon is found in minerals: chalk, marble, limestone - CaCO3, dolomite - MgCO3?CaCO3; hydrocarbonates - Mg (HCO3) 2 and Ca (HCO3) 2, CO2 is part of the air; carbon is the main component of natural organic compounds - gas, oil, coal, peat, is part of organic substances, proteins, fats, carbohydrates, amino acids that are part of living organisms.
Phosphoric anhydride
| Phosphorus(V) oxide | |
 |
|
| General | |
|---|---|
| Systematic name | Phosphorus(V) oxide |
| Chemical formula | P2O5 |
| Rel. molek. weight | 283.889 a. eat. |
| Molar mass | 283.889 g/mol |
| Physical properties | |
| Matter density | 2.39 g/cm³ |
| Condition (st. conv.) | White powder |
| Thermal Properties | |
| The melting temperature | 420 o C (H-form), 569 (O-form) ° C |
| Boiling temperature | sublimes at 359 (H-form) °C |
| Enthalpy (st. arb.) | -3010.1 kJ/mol |
| Chemical properties | |
| Solubility in water | reacts g/100 ml |
| Classification | |
| CAS number | (P2O5) (P 4 O 10) |
Phosphorus pentoxide (phosphoric anhydride, phosphorus pentoxide, oxide (V) phosphorus- P 2 O 5 , acid oxide.
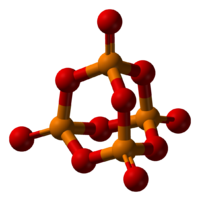
Structure
Pairs of phosphorus oxide (V) have the composition P 4 O 10 . Solid oxide is prone to polymorphism. Exists in an amorphous glassy state and a crystalline state. For the crystalline state, two metastable modifications of phosphorus pentoxide are known - the hexagonal H-form (a = 0.744 nm, = 87 °, space, gr. R3C) and the orthorhombic O-form (a = 0.923 nm, b - 0.718 nm, c = 0.494 nm , spaces, gr. Ppat), as well as one stable orthorhombic O-form (a \u003d 1.63 nm, b \u003d 0.814 nm, c \u003d 0.526 nm, space group Fdd2). Molecules P 4 O 10 (H-form) are built from 4 groups PO 4 in the form of a tetrahedron, the vertices of which are occupied by phosphorus atoms, 6 oxygen atoms are located along the edges, and 4 - along the threefold axis of the tetrahedron. This modification easily sublimates (360 ° C) and actively interacts with water.
Other modifications have a layered polymer structure, also built from PO 4 tetrahedra, combined into 10-membered (O-form) and 6-membered (O "form) rings. These modifications have more high temperature sublimations (~580 o C) and less chemically active. The H-form passes into the O-form at 300-360 o C.
Properties
P 4 O 10 interacts very actively with water (the H-form absorbs water even with an explosion), forming mixtures of phosphoric acids, the composition of which depends on the amount of water and other conditions:
P 4 O 10 + 6H 2 O (l) → 4H 3 PO 4 (-177 kJ)
It is also capable of extracting water from other compounds, making it a powerful dehydrator:
2HNO 3 + P 2 O 5 → 2HPO 3 + N 2 O 5; 4HClO 4 + P 4 O 10 → (HPO 3) 4 + 2Cl 2 O 7.
Phosphorus (V) oxide is widely used in organic synthesis. It reacts with amides, converting them to nitriles:
P 4 O 10 + RC (O) NH 2 → P 4 O 9 (OH) 2 + RCN P 4 O 10 + RCO 2 H → P 4 O 9 (OH) 2 + 2 O
Also interacts with alcohols, ethers, phenols and others organic compounds. In doing so, a break occurs P-O-P connections and organophosphorus compounds are formed. Reacts with NH3 and with hydrogen halides to form ammonium phosphates and phosphorus oxyhalides:
P 4 O 10 + 8PCl 3 + O 2 → 12Cl 3 PO
When P 4 O 10 is fused with basic oxides, it forms various solid phosphates, the nature of which depends on the reaction conditions.
Receipt
Phosphorus(V) oxide is obtained by burning phosphorus. Technological process takes place in the combustion chamber and includes the oxidation of elemental P with pre-dried air, the precipitation of P 4 O 10 and the purification of exhaust gases. The resulting pentoxide is purified by sublimation.
P 4 + 5O 2 → P 4 O 10 + 2984 kJ.
The technical product has the appearance of a white snow-like mass, consisting of a mixture different forms P 4 O 10 .
Application
P 4 O 10 is used as a dryer for gases and liquids. It is also an intermediate product in the production of phosphoric acid H 3 PO 4 by the thermal method.
It is widely used in organic synthesis in dehydration and condensation reactions.
Literature
- Akhmetov N. S. "General and inorganic chemistry" M .: Higher school, 2001
- Remy G. "Course of inorganic chemistry" M .: Foreign Literature, 1963
- F. Cotton, J. Wilkinson "Modern inorganic chemistry" M.: Mir, 1969
Wikimedia Foundation. 2010 .
See what "Phosphoric anhydride" is in other dictionaries:
PHOSPHORIC ANHYDRIDE- (p2o5) phosphorus oxide V (see); white powder, very hygroscopic, forms phosphorus with water (see); used as a strong desiccant for gases and liquids. In case of contact with the skin and an attempt to wash it off with water, a strong thermochemical is guaranteed. burn. At… … Great Polytechnic Encyclopedia


