How to find spring extension if mass is known. The concept of water hardness, calculation formula, units of measurement, norms in Russia
The fact that tap water is too hard must be heard constantly. What measures the "hardness" of a liquid? And how water hardness is calculated.
In the photo: heating element “affected” by hardness salts
If scientific reasoning about the combination of fluid properties is minimized, then the total amount of dissolved salts of alkaline earth metals (mainly calcium and magnesium) can be considered water hardness. From a chemical point of view, all divalent cations, to one degree or another, are able to bind to anions, forming salts that can precipitate. However, in practice, the amount of strontium, barium, and manganese dissolved in water is minimized, and aluminum and iron transfer to salt complexes only at a certain level of acidity of the medium (pH less than 7), which practically does not occur in nature. We already wrote about how to determine stiffness at home.
Table 1. Cations and anions that cause stiffness
|
Hardness Cations |
Stiffness anions |
|
Calcium (Ca 2+) |
Bicarbonate (NSO 3 -) |
|
Magnesium (Mg 2+) |
Sulfate (SO 4 2-) |
|
Strontium (Sr 2-) |
Chloride (Cl -) |
|
Iron (Fe 2+) |
Nitrate (No. 3 -) |
|
Manganese (Mn 2+) |
Silicate (Sio 3 2-) |
Water hardness formula
Carbonate hardness is the quantitative content of magnesium and calcium hydrogen carbonates and carbonates (MgHCO3, CaHCO3) in water. This type of pollutant is easily eliminated by boiling with the formation of carbonic acid and sediment:
Ca 2+ + 2HCO 3 - (when heated) \u003d CaCO 3 ↓ + H 2 O + CO 2
Non-carbonate (constant) hardness is due to the presence of magnesium and calcium compounds of strong acids (nitric, hydrochloric, sulfuric). When boiling, salts of this type do not decompose.
The formula for calculating the total hardness of water: H total \u003d N carb + N non-carb
Units
Units of measure in Russia - degrees (° F), can be expressed in volume or mass fraction. 1 degree of hardness is numerically equal to 0.5 molar concentration of alkaline earth element, expressed in mg / cu. dm. (1 ° W \u003d 1 mEq / L.)
SI units are mol / cubic meter. However, in practice, most often used mEq / L Concentration attributed to a unit of mass justifies itself in those cases when it is necessary to analyze water in a different state of aggregation (with a changed density).
For those to whom chemistry was not easy at lessons, it is necessary to repeat:
One mEq / L corresponds to 20.04 milligrams of ionsCa 2+ or 12.16 milligrams of ionsMg 2+ (ratio of atomic mass and valency of an element).
Stiffness standards
In terms of hardness, water can be divided into three categories:
Soft (up to 2 ° W),
Medium hardness (2-10 ° W),
Very tough (more than 10 ° W).
Rigidity standards in Russia do not exceed 7 mEq / L. According to EU standards, the MPC for the total hardness of water cannot be more than 1.2 mEq / l. Using simple calculations, we can conclude that in Europe, water is almost 6 times softer than in Russia.
Thus, the numerically confirmed need to install cleaning systems in Russia is no longer in doubt. In addition, according to internationally accepted standards, the water used by our compatriots needs multistage softening and deep filtration.
Now let's figure out how to eliminate excessive salt content in water:
Used Books:
- GOST 2874-82
- GOST R 52029-2003 | NATIONAL STANDARDS
- Chemical encyclopedia. - M.: Soviet Encyclopedia, 1990.V. 2.P. 145.
14.1. Hardness of water
Water hardness - a combination of chemical and physical properties of water associated with the content of dissolved metal salts in it, mainly calcium Ca 2+ and magnesium Mg 2+ (the so-called "hardness salts"). There are temporary (carbonate) stiffness caused by calcium and magnesium bicarbonates (Ca (НСО 3) 2; Mg (НСО 3) 2), and constant (non-carbonate) stiffness caused by the presence of other salts that are not released during boiling water: mainly sulfates and Ca and Mg chlorides (CaSO 4, CaCl 2, MgSO 4, MgCl 2). The total hardness of water is equal to the sum of the temporary (carbonate) and constant (non-carbonate) hardness.
In the SI system, stiffness is measured in mol / m 3. In practice, degrees of rigidity are used, stiffness is expressed in milligrams of equivalents per liter, and also in millimole equivalents per liter. 1 ° W corresponds to a concentration of alkaline earth element, numerically equal to 1/2 of its mole per liter (1 ° W \u003d 1 mEq / l \u003d 1/2 mmol-eq / l). The total hardness is distinguished by soft water (up to 2 ° F), medium hardness (2-10 ° F) and hard water (more than 10 ° F).
In this manual, 1 mEq / L is taken as the dimension of water hardness (often the equivalent is passed and water hardness is expressed in mmol / L), which is expressed by the sum of millimole (mmol) equivalents of Ca 2+ and Mg 2+ ions contained in 1 liter of water. One millimole of hardness corresponds to a content of 20.04 mg / L Ca 2+ and 12.16 mg / L Mg 2+.
Water hardness can be calculated by the formula presented in general form:
where W is the water hardness, mmol-equiv / l; m i is the mass of cations (or corresponding salts), mg; M Ek i is the molar mass of equivalents of cations (or their corresponding salts), mg / mmol; V - water volume, l
1. Determination of stiffness by weight of salts contained in it.
Example 1 Calculate the total hardness of the water (mmol / L) if 0.20 L of water contains 32.42 mg of calcium bicarbonate Ca (HCO 3) 2; 1.46 mg of magnesium bicarbonate Mg (HCO 3) 2; 22.20 mg of calcium chloride CaCl 2 and 4.75 mg of magnesium chloride MgCl 2.
Decision. The total hardness of water can be calculated by the formula (1)
Masses of salts and volume of water are known by the condition of the problem. We find the molar masses of salt equivalents. The molar mass of salt equivalents is equal to the molar mass of salt divided by the equivalent number Z. For all salts Z equal to 2.
M Ek Ca (NSO 3) 2 \u003d M Ca (HCO 3) 2/2 \u003d 162.11 / 2 - 81.05 mg / mmol;
M ec Mg (HCO 3) 2 \u003d M Mg (HCO 3) 2/2 \u003d 146.34 / 2 \u003d 73.17 mg / mmol;
M ec CaCl 2 \u003d M CaCl 2/2 \u003d 110.99 / 2 \u003d 55.49 mg / mmol;
M ec MgCl 2 \u003d M MgCl 2/2 - 95.21 / 2 \u003d 47.60 mg / mmol.
Substituting the masses, molar masses of salt equivalents and the volume of water in the formula, we calculate the total water hardness:
2.0 + 0.1 + 2.0 + 0.5 \u003d 4.6 mmol / L.
Example 2 Calculate the temporary hardness of the water, knowing that 500 l of it contains 162.1 g of Ca (HCO 3) 2.
Decision. The temporary hardness of water can be found by formula (1), substituting the mass, molar mass of Ca equivalents (НСО 3) 2 and the volume of water into it. The molar mass of Ca equivalents (HCO3) 2 is 81.05 mg / mmol (see Example 1), the mass of this salt is 162100 mg, the volume of water is 500 l. Consequently,
2. Determination of salt content in water by water hardness
Example 3. How many grams of CaCl 2 are contained in 100 l of water if the hardness of the water caused by this salt is 2.5 mmol / l?
Decision. The mass of CaCl 2 salt can be found by the formula (1). Namely:
Substituting the water hardness, the molar mass of CaCl 2 equivalents (see Example 1) and the volume of water in the formula, we obtain:
M CaCl 2 \u003d 2.5 · 55.49 · 100 \u003d 13872.5 mg or 13.8725 g.
3.Determination of the temporary (carbonate) hardness of water by the volume of hydrochloric acid used for its titration
The simplest method for determining water hardness is the titration method. To determine the temporary hardness of water using a standard solution of hydrochloric acid. In this case, the following reaction occurs:
Me (НСО 3) 2 + 2 НС1 \u003d МеС1 2 + 2 Н 2 О + 2СО 2,
where Me is Ca 2+, Mg 2+, Fe 2+.
According to the law of equivalents, the number of equivalents of all substances involved in a chemical reaction must be the same. From here:
where C ec1 and V 1 - respectively, the molar concentration of the equivalents of the substance (mol / l) and volume (l) of the first solution; C ec2 and V 2 are the molar concentration of the equivalents of the substance (mol / l) and the volume (l) of the second solution.
Example 4. Determine the temporary hardness of the water if a titration of 5 · 10 –2 L of water containing calcium bicarbonate consumed 1.44 · 10 –3 L of a 1.15 N HCl solution.
Decision. According to the condition of the problem, the molar concentration of equivalents of Ca (HCO 3) 2 is unknown. We denote it by x. Substituting the values \u200b\u200bin formula (2), we obtain:
x · 5 · 10 –2 \u003d 1.44 · 10 –3 · 0.15.
Thus, the molar concentration of equivalents of Ca (HCO 3) 2 is 0.004 N, which corresponds to a content of Ca (HCO 3) 2 of 0.004 mol / L or 4 mmol / L. Therefore, the temporary hardness of water is 4 mmol / L.
Example 5. What is the temporary hardness of water equal to if 5.75 ml of a 0.07 N HCl solution was consumed in the titration of 100 ml of this water containing iron (II) bicarbonate.
Decision. This problem is solved in the same way as shown in example 5, previously converting the volume of solutions in liters, i.e. V 1 \u003d 0.1 L H 2 O; V 2 \u003d 5.75 · 10 –3 L of HCl. Substituting the values \u200b\u200bin the formula (2), we obtain:
x · 0.1 \u003d 5.75 · 10 –3 · 0.07.
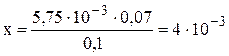 n
n
The molar concentration of equivalents of Fe (HCO 3) 2 corresponds to the content of this salt equal to 4 · 10 –3 mol / L or 4 mmol / L.
Therefore, the water hardness is 4 mmol / L.
14.2. Water softening methods
To soften the water, precipitation and ion exchange methods are used. By precipitation, Ca 2+ and Mg 2+ cations are precipitated into sparingly soluble compounds. This can be achieved by boiling or chemically by introducing into the water, for example, soda Na 2 CO 3, slaked lime Ca (OH) 2, etc. When boiling, only temporary (carbonate) stiffness according to the reaction is eliminated:
Ca (HCO 3) 2 CaCO 3 ¯ + CO 2 + H 2 O.
Mg (HCO 3) 2 Mg (OH) 2 ¯ + CO 2.
Upon decomposition of Mg (HCO 3) 2, Mg (OH) 2 is formed, not MgCO 3, since
The method of chemical softening is based on adding soda ash Na 2 CO 3 or slaked lime Ca (OH) 2 to the water. In this case, calcium and magnesium salts pass into insoluble compounds and, as a result, precipitate. For example, the addition of slaked lime results in the conversion of calcium salts to insoluble carbonate:
Ca (HCO 3) 2 + Ca (OH) 2 → 2 CaCO 3 ↓ + 2H 2 O
The best reagent for eliminating the general hardness of water is sodium orthophosphate Na 3 PO 4:
3Ca (HCO 3) 2 + 2Na 3 PO 4 → Ca 3 (PO 4) 2 ↓ + 6NaHCO 3
3MgSO 4 + 2Na 3 PO 4 → Mg 3 (PO 4) 2 ↓ + 3Na 2 SO 4
Orthophosphates of calcium and magnesium are very poorly soluble in water, so they are easily separated by mechanical filtration.
The ion exchange method is based on the use of granular ion-exchange resin, which absorbs cations of hardness salts (calcium and magnesium, iron and manganese). In exchange, depending on the ionic form, the resin gives off sodium or hydrogen ions. These methods are respectively called Na-cation and H-cation.
4.Determination of the mass of the reagent necessary to eliminate the hardness of the water.
Example 6 How many grams of soda Na 2 CO 3 must be added to 150 liters of water to remove a hardness of 5 mmol / l?
Decision. 150 l of water contains 150 · 5 \u003d 750 mmol / l of salts, causing water hardness. According to the law of equivalents, in order to eliminate this stiffness, it is necessary to add the same amount of substance equivalents that soften water, i.e. 750 mmol Na 2 CO 3.
To find the substance in grams, the amount of substance equivalents is multiplied by the molar mass of equivalents of this substance. In this case, the equivalent number of Na 2 CO 3 is equal to two, and the molar mass of equivalents of Na 2 CO 3 will be
M ec Na 2 CO 3 \u003d M Na 2 CO 3/2 \u003d 106/2 \u003d 53 mg / mmol.
Find the mass of Na 2 CO 3:
m Na 2 CO 3 \u003d n ec Na 2 CO 3 M ec Na 2 CO 3 \u003d 750 · 53 \u003d 39 750 mg or 39.75 g
The same problem can be solved using formula (1). For this, it must be remembered that according to the law of equivalents, the masses of reacting substances are proportional to their molar masses of equivalents. Therefore, in the formula (1) instead of the molar mass of equivalents of salts that cause rigidity, you can substitute the molar mass of equivalents of substances necessary to eliminate this stiffness in order to find their mass. In this case:
m Na 2 CO 3 \u003d W · M ek Na 2 CO 3 · V.
Substituting the values \u200b\u200bof W, M ek Na 2 CO 3 and V, we obtain:
m Na 2 CO 3 \u003d 5 · 53 · 150 \u003d 39750 mg or 39.75 g.
CONTROL TASKS
261. What is meant by water hardness? What is the hardness of water measured? 1 liter water contains 38.0 mg of calcium bicarbonate and 19.6 mg of magnesium sulfate. What is the total hardness of water equal to? What hardness group does such water belong to?
Answer: 0.8 mmol / L.
262. Why is it necessary to eliminate excessive water hardness? What is the danger of such water? Give examples. What is the hardness of water containing 0.01 mol / L calcium chloride equal? What hardness group does such water belong to?
Answer: 20 mmol / L.
263. What determines the constant hardness of water? What chemical methods exist to eliminate the constant hardness of water? Write the equations of the corresponding reactions. What is the constant hardness of water equal to if 2.5 l of it contains 40 mg of calcium sulfate? What hardness group does such water belong to?
Answer: 0.23 mmol / L.
264. What is the total hardness of water made of? Explain the answer. What is the total hardness of water equal to if 57 mg of magnesium bicarbonate and 33 mg of calcium sulfate are in 3.4 liters of water? What hardness group does such water belong to?
Answer: 0.37 mmol / L.
265. What causes the temporary hardness of water? What methods can eliminate excessive temporary water hardness? Write the necessary equations of chemical reactions. When boiling 0.25 liters of water containing only calcium bicarbonate, 4 mg of calcium carbonate precipitates. What is the temporary hardness of water equal to? What hardness group does such water belong to?
Answer: 0.32 mmol / L.
266. What determines the constant hardness of water? What chemical methods exist to eliminate the constant hardness of water? Write the equations of the corresponding reactions. How many grams of magnesium sulfate are contained in 150 l of water if the hardness of the water caused by this salt is 4.7 mmol / l? What hardness group does such water belong to?
Answer: 42.441 g.
267. What determines the temporary hardness of water? What methods can eliminate excessive temporary water hardness? Write the necessary equations of chemical reactions. When boiling 0.5 l of water containing only iron (II) bicarbonate. 8 mg of iron (II) carbonate precipitates. What is the temporary hardness of water equal to? What hardness group does such water belong to?
Answer: 0.28 mmol / L.
268. What is titration? What law is the titration method based on? Give the wording of this law. For titration of 25 mg of water, 2.4 ml of a 0.1N HC1 solution was consumed. What is the carbonate hardness of water equal to? What hardness group does such water belong to? What hardness group does such water belong to?
Answer: 9.6 mmol / L.
269. What is the total hardness of water made of? Explain the answer. What methods can eliminate excessive total hardness of water? Write the appropriate reaction equations. To soften 200 l of water, 12.72 g of sodium carbonate was required. What is the hardness of water equal to? What hardness group does such water belong to?
Answer: 1.2mmol / L.
270. What determines the temporary hardness of water? What methods can eliminate excessive temporary water hardness? Write the necessary equations of chemical reactions. The temporary hardness of water is 6.64 mmol / L. What weight of calcium hydroxide must be taken to remove the hardness of 10 liters of water.
Answer: 2,459g.
271. What chemical methods exist to eliminate excess water hardness? Write the corresponding equations of the reactions proceeding at softening of water. How many grams of sodium orthophosphate must be added to 250 liters of water to eliminate its carbonate hardness of 2.5 mmol / l? What hardness group does such water belong to?
Answer: 34.162 g.
272. What is titration? What law is the titration method based on? Give the wording of this law. For titration of 40 ml of water, 5.7 ml of 0.12 N was consumed. HC1 solution. What is the carbonate hardness of water equal to? What hardness group does such water belong to?
Answer: 20 mmol / L.
273. What determines the constant hardness of water? What chemical methods exist to eliminate the constant hardness of water? Write the equations of the corresponding reactions. 500 g of water contains 70 g of calcium sulfate. What is the constant hardness of this water? What hardness group does such water belong to?
Answer: 2.1 mmol / L.
274. What is the total hardness of water made of? Explain the answer. What methods can eliminate excessive total hardness of water? Write the appropriate reaction equations. What is the total hardness of water equal if 32.85 g of calcium bicarbonate and 30.6 g of magnesium sulfate are contained in 300 l of it? What hardness group does such water belong to?
Answer: 3.1 mmol / L.
275. What determines the temporary hardness of water? What methods can eliminate excessive temporary water hardness? Write the necessary equations of chemical reactions. Water containing only calcium bicarbonate has a hardness of 4.2 mmol / L. What hardness group does such water belong to? How many grams of calcium bicarbonate are in 250 liters of water?
Answer: 85.102 g.
276. Why is it necessary to eliminate excessive water hardness? What is the danger of such water? Give examples. To 280 L of hard water, 62.5 g of sodium carbonate was added. Write down the equations for the possible reactions. Calculate how much mmol / L water hardness has decreased?
Answer: 4 mmol / L.
277. What determines the temporary hardness of water? What methods can eliminate excessive temporary water hardness? Write the necessary equations of chemical reactions. Water containing only magnesium bicarbonate has a hardness of 7.8 mmol / L. What hardness group does such water belong to? What is the mass of magnesium bicarbonate in 350 liters of water?
Answer: 221.266 g.
278. What is meant by water hardness? What is the hardness of water measured? What mass of calcium hydroxide should be added to 150 liters of water to eliminate the temporary hardness of water equal to 2.5 mmol / l? What hardness group does such water belong to?
Answer: 13.89 g.
279. What is titration? What law is the titration method based on? Give the wording of this law. Titration of 40 ml of water required 3.85 ml of 0.15 K HC1 solution. What is the carbonate hardness of water equal to? What hardness group does such water belong to?
Answer: 14.4 mmol / L.
280. What determines the constant hardness of water? What chemical methods exist to eliminate the constant hardness of water? Write the equations of the corresponding reactions. What is the hardness of water equal to 10 l of which contains 0.025 mol of magnesium sulfate? What hardness group does such water belong to?
13. RIGIDITY
Water hardness is a property of natural water, depending on the presence of mainly dissolved calcium and magnesium salts in it. The total content of these salts in water in mmol / dm 3 is called the total hardness.
The main sources of calcium and magnesium in surface waters are the processes of chemical weathering and dissolution of minerals, primarily limestones and dolomites. Significant amounts of calcium and magnesium ions can enter water bodies with sewage from silicate, metallurgical, textile, glass, chemical and other industries.
The ionic form of calcium and magnesium is characteristic of low-saline waters. A significant part of them is in the form of neutral or charged forms () of ion pairs, as well as water bound in complexes with organic substances.
The total stiffness ranges from units to tens, sometimes hundreds of mmol / dm 3. Usually prevails (up to 70%) stiffness due to calcium ions.
The hardness of sea water and ocean water is usually higher, and often magnesium hardness is superior to calcium.
The total hardness of surface waters is subject to noticeable seasonal fluctuations, usually reaching the highest values \u200b\u200bat the end of winter and the lowest during floods. Groundwater hardness is more constant.
High rigidity, especially due to magnesium salts, impairs the organoleptic properties of water, giving it a bitter taste, and has an effect on the digestive organs. Depending on the pH and alkalinity of the water, hardness above 10 mmol / dm 3 can cause slag formation in the distribution system of water supply and scale during heating.
13.1. Determination of total water hardness
complexometric method
Method principle. The method is based on the formation of complex compounds of Trilon B with ions of alkaline earth elements. The determination is carried out by titration of a sample with a solution of Trilon B at pH 10 in the presence of an eriochrome black T.
At pH 10, Trilon B is able to form slightly dissociated complexes with magnesium and calcium ions:
Na 2 H 2 Y + Ca 2+ ↔ Na 2 CaY + 2H +;
Na 2 H 2 Y + Mg 2+ ↔ Na 2 MgY + 2H +.
The eriochrome black T indicator (special black chromogen ET-100), when added to the sample, forms a red-violet complex compound with magnesium ions. During titration, Trilon B combines with calcium ions, and then with magnesium ions and displaces the indicator, which in free form has a blue color. At the equivalence point, the indicator gives a sharp transition in color:
HInd 2- + Mg 2+ ↔ MgInd - + H +
red violet
MgInd - + Na 2 H 2 Y ↔ Na 2 MgY + HInd 2- + H +.
red violet
Ca 2+ ions do not give such a clear change in the color of the indicator, and therefore, Ca 2+ ions cannot be determined separately in the presence of the Eriochrom black T indicator.
To obtain reproducible results, it is necessary that the titrated solution has a pH \u003d 10 ± 0.2 and a sufficient amount of magnesium ions.
If the test sample was acidified for preservation or the sample has an acidic medium, then a solution of sodium hydroxide with a molar concentration of 0.2 mol / dm 3 to pH 6 - 7 is added to an aliquot of the sample. If the water sample has a strongly alkaline medium, then a saline solution is added to the aliquot. acid molar concentration of 0.1 mol / DM 3 to pH 6 - 7. The pH control is carried out on a universal indicator paper or using a pH meter.
Elimination of interfering influences.The presence of more than 10 mg / dm 3 iron ions in water, more than 0.05 mg / dm 3 of each of copper, cadmium, cobalt, lead ions, more than 0.1 mg / dm 3 of each of manganese (II) ions, aluminum, zinc , nickel, tin, as well as color more than 200º and increased turbidity during titration cause a fuzzy color change at the equivalence point, and leads to an overestimation of the stiffness determination results. Orthophosphate and carbonate ions can precipitate calcium under titration conditions at pH 10. The interfering effect of Zn 2+ ions is up to 200 mg / dm 3; Al 3+, Cd 2+, Pb 2+ up to 20 mg / dm 3; Fe 3+ up to 5 mg / dm 3; Mn 2+, Co 2+, Cu 2+, Ni 2+ up to 1 mg / dm 3 are eliminated by adding sodium sulfide solution to the titrated sample with a mass fraction of 0.05 (5%). To reduce the effect of manganese to 1 mg / dm 3, iron, aluminum to 20 mg / dm 3, copper to 0.3 mg / dm 3 add from 5 to 10 drops of a solution of hydroxylamine hydrochloride. The turbidity of the sample is eliminated by filtration through membrane filters with pores with a diameter of 0.45 μm or paper anesthetized filters "blue tape".
If it is impossible to eliminate the interfering effect, then the stiffness is determined by atomic spectroscopy.
Selection of sample volume for analysis. Before analyzing a water sample with an unknown stiffness value, an evaluation titration is performed. To do this, take 10 cm 3 of water, add 0.5 cm 3 of buffer solution, indicator and titrate with Trilon B solution with from(1/2 Na 2 H 2 Y) \u003d 0.05 mol / dm 3 until the color turns blue. The value of the volume of trilon B solution used for titration is selected from the table. 13.1 appropriate sample volume of water.
The course of determination. The required sample volume is pipetted into a conical flask with a capacity of 250 cm 3, adjusted, if necessary, to 100 cm 3 with distilled water, 5 cm 3 of buffer solution is added, from 0.05 to 0.1 g of dry indicator mixture. The sample is thoroughly mixed and titrated with Trilon B solution from(1/2 Na 2 H 2 Y) \u003d 0.05 mol / dm 3 until the red-violet color turns to blue. Titration is repeated and, if the difference between parallel titrations does not exceed 0.05 cm 3 with a volume of Trilon B solution of 5 cm 3 or less than 0.1 cm 3 with a volume of more than 5 cm 3, the average value of the volume of Trilon B solution is taken as the result. Otherwise titration is repeated until an acceptable discrepancy is obtained.
Payment. Total water hardness X, mmol / dm 3, calculated by the formula![]()
where from(1/2 Na 2 H 2 Y) is the molar concentration of the equivalent of Trilon B, mol / dm 3;
V(Na 2 H 2 Y) - the volume of Trilon B solution, which went to the titration of the sample, cm 3;
V
13.2. Determination of the mass concentration of calcium and magnesium ions by the complexometric method. Calculation of water hardness in degrees of hardness (ºЖ)
The method is based on the ability of calcium ions to form stable complexes with Trilon B in a strongly alkaline medium (pH \u003d 12 - 13). A similar complex of magnesium ions in this medium is destroyed with the release of magnesium hydroxide. When titrated with Trilon B solution, the color change of the indicator (murexide) from lilac to red-raspberry indicates complete binding of calcium ions:
H 2 Ind 3- + Ca 2+ ↔ CaH 2 Ind -;
red raspberry
CaH 2 Ind - + Na 2 H 2 Y ↔ Na 2 CaY + H 2 Ind 3- + 2H +.
Titration of calcium ions is possible with the joint presence of heavy metal ions in concentrations not exceeding the values: for copper - 0.2 mg / dm 3; zinc, lead, nickel, manganese, iron, aluminum - 1 mg / dm 3 and magnesium - 3 mg in a determined volume. At higher concentrations of heavy metal ions, sodium sulfide is added to the sample. The interfering effect of magnesium ions is eliminated either by reducing the volume of the sample taken for analysis, or with a high magnesium content (Mg: Ca ratio of more than 1), precipitation of magnesium ions with a sodium hydroxide solution having a concentration of 2 mol / dm 3 (pH 12 - 13) in the measured flask with a capacity of 100 cm 3. For this, 20–40 cm 3 of the sample are diluted with distilled water to 90 cm 3 and NaOH solution is slowly added dropwise, mixed well, while a small amount of calcium ions are precipitated with Mg (OH) 2. The volume of the solution was adjusted to the mark with distilled water, and after settling the precipitate for 1.5 - 2 hours, a transparent aliquot was selected for titration. To reduce calcium loss, the settling time should not exceed 2 hours.
The course of determination. The required sample volume is measured in a conical flask with a capacity of 250 cm 3, adjusted, if necessary, to 100 cm 3 with distilled water, add 2 cm 3 NaOH solution with a mass fraction of 0.08 (8%), 0.1 - 0.2 g of indicator murekida and is slowly titrated with Trilon B solution with vigorous stirring until the color changes from red-crimson to purple. The titration is repeated and, if the discrepancy between the parallel titrations does not exceed the values \u200b\u200bgiven in the table. 13.2, the average value of Trilon B is taken as the result. Otherwise, titration is repeated until an acceptable discrepancy is obtained.
Payment. The mass concentration and amount of the substance of the equivalent of calcium ions in the analyzed water sample is found by the formulas:
where m(Ca 2+) - mass concentration of calcium ions in water, mg / dm 3;
n(1 / 2Ca 2+) - the amount of the substance is the equivalent of calcium ions in water, mmol / dm 3;
![]() c(1 / 2Na 2 H 2 Y) is the molar concentration of the equivalent of trilon B, mol / dm 3;
c(1 / 2Na 2 H 2 Y) is the molar concentration of the equivalent of trilon B, mol / dm 3;
V(Na 2 H 2 Y) - the volume of Trilon B solution, which went to the titration of the sample, cm 3;
M(1 / 2Ca 2+) is the molar mass of the equivalent of calcium ions, mg / mmol;
V - the volume of water samples taken for determination, cm 3.
The mass concentration of magnesium ions in mg / DM 3 in the analyzed water sample is determined by the formula
where m(Mg 2+) - mass concentration of magnesium ions in water, mg / dm 3;
X - total water hardness, mmol / dm 3;
n(1 / 2Ca 2+) - the amount of the substance is the equivalent of calcium ions in water, mmol / dm 3;
M(1 / 2Mg 2+) is the molar mass of the equivalent of Mg 2+, mg / mmol.
Table 13.2
Acceptable discrepancies between parallel titrations depending on the volume of Trilon B solution
In accordance with GOST R 52029-2003, water hardness is expressed in degrees of hardness (ºЖ).
The hardness degree corresponds to the concentration of the alkaline earth element, numerically equal to 1/2 mole, expressed in mg / dm 3 (g / m 3).
The hardness of water W, ºF, with a separate quantitative determination of ions of alkaline earth elements is calculated by the formula
![]()
where m(Ca 2+) is the mass of calcium in the water sample, mg / dm 3;
m(Mg 2+) is the mass of magnesium in the water sample, mg / dm 3;
M(Ca 2+) - molar mass of calcium, mg / mol;
M(Mg 2+) - molar mass of magnesium, mg / mol.


