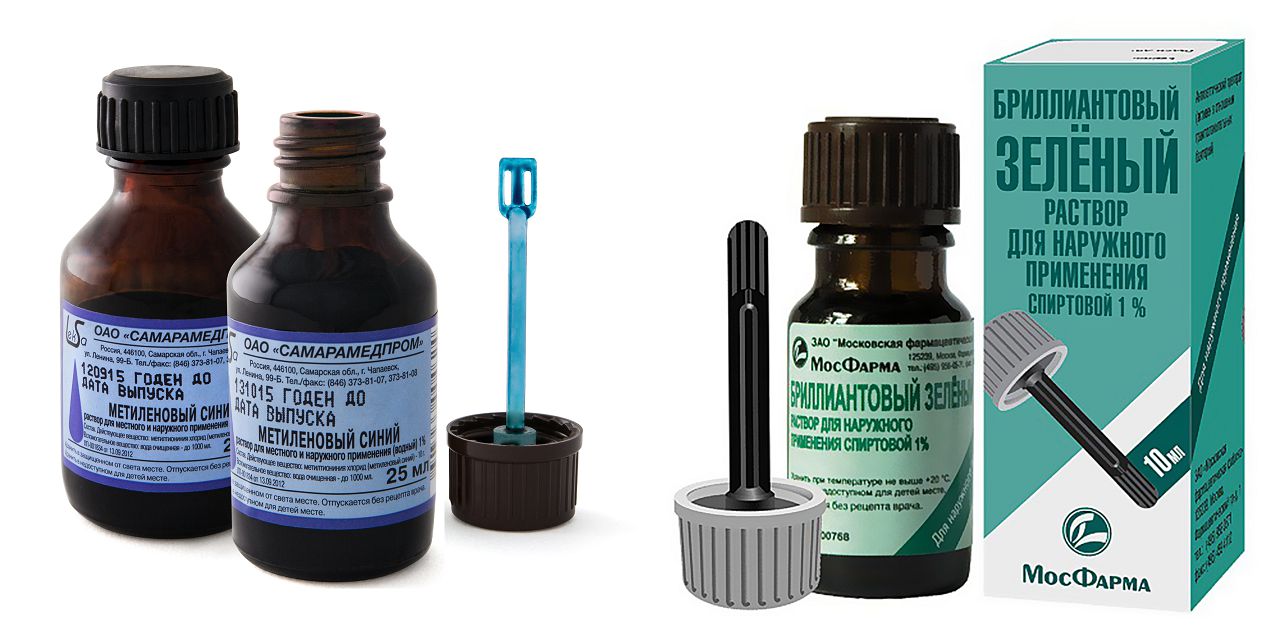
Dye methylene blue instruction. Methylene blue
Hello, dear listeners! Blue iodine is not an innovation, but a forgotten old remedy. Almost a century ago, it was used to treat dysentery, which at that time killed thousands of people. Later, the mixture was used in the fight against angina, ulcers, the effects of stroke, etc. So where did yodinol from pharmacies go? He suffered the fate of potassium permanganate: permanent bans and restrictions. Of course, it is more profitable for pharmacists to sell you a whole expensive package of medicines, and not just one cheap one. Then we will do our own health! Recall what kind of animal it is - blue medical instructions for use, properties and methods of preparation.
Edible iodine
Sounds crazy, right? What is the difference between blue and your brother? The usual means can not be taken orally, even in the form of tinctures. If it burns the skin, then everything inside is all the more so. However, the properties of iodine seemed quite promising, and the scientist V. O. Mokhnach invented a way to make it safe. It's very simple: you just need to add starch! Antimicrobial properties are enhanced, and the additive neutralizes the action of toxins: it creates a protective layer. The mixture is even administered intravenously with severe poisoning.
Let's find out the usefulness of iodinol.
- Kills bacteria and germs. Copes with any infection from tonsillitis to conjunctivitis.
- Cleans the blood and blood vessels. The level of cholesterol and sugar decreases, the number of erythrocytes increases.
- Normalizes pressure and makes the walls of blood vessels elastic.
- Dramatically improves the immune system. Helps the body to fight most diseases.
- Treats burns.
And this is not all its qualities, only the main ones. It is important to know how to properly use methylene blue, because it is still a concentrated medicine.
We use wisely

There is no need to drink medical bluing in liters. Do not forget that such a shock dose can harm the thyroid gland. The list of exceptions includes only a few cases. Let's talk about them and about the standard methods of application.
1. Prevention
Are you afraid of iodine deficiency? If you do not get the norm of substances, just drink 1 tsp. methylene blue twice a week. It'll be enough. The whole course will take a month, you can repeat it in six months. During seasonal epidemics, it is permissible to increase the dose to 3 spoons per week.
2. Dysentery
During the day, drink 0.5 liters of fluid. Take a little, literally a tablespoon. After a few days, the condition returns to normal.
3. Acute poisoning
Saves, for example, with vinegar intoxication. During the day you need to take up to 2 liters in portions of 100 ml. The treatment will take several days.
4. Ulcer
Dose - 1 l. Daily prepare a new batch. Add more syrup to make it easier to drink. During the day, use the medicine a few spoons before meals. In the evening, drink the rest. Course duration - a month. The tool helps even when the stomach has become similar to a sieve.
5. Stomatitis
The child simply irrigate the oral cavity. Adults should do applications. Usually, recovery occurs on the 3rd day.
6. Inflammation of the gums
Mix methylene blue with water (1: 1) and rinse your mouth twice a day. After about 2 days the inflammation will pass.
7. Candidiasis
Killing the fungus is incredibly difficult. Often medicines destroy not only the pathogen, but at the same time the entire gastrointestinal tract. Medical blue is much safer in this regard. To get rid of the fungus, you need to take orally 50 ml three times a day and spray 30 ml into the oral cavity. Gradually the growths will disappear and the mucosa will return to normal.
8. Angina
With a concentrated solution, gargle every hour. After the procedure, 2-3 times a day, drink 3 sips.
How to prepare your own medicine
The best pill is the one you made with your own hands. In addition, the purchase of finished medical bluing is now not so easy. Of course, you can kill the day to search for iodinol, but it is better to cook it for 5-10 minutes.
You will need:
- 1 tsp starch (10 g)
- 1 tsp sugar (10 g)
- several crystals of citric acid (0.4 g)
- 1 tsp 5% alcohol solution of iodine.
Please do not neglect the components! We need lemon for acidification so that starch does not lose its properties. Sugar partially neutralizes toxins and improves taste. If you plan to give medicine to a child, you can add more sugar or fruit syrup.
- Dissolve starch in 50 ml of water
- Add the remaining solids.
- Bring to a boil 150 ml of water. Slowly pour in the blank
- Remove the mixture from the heat. Let her cool
- Pour in iodine.
It remains only to observe the change in color. The miracles of transformation!
Contraindications
Do not take medicine for lactating and pregnant women, as well as young children up to a year. Be sure to follow the dosage. In large volumes, the remedy is drunk only with severe intoxication, dysentery, and some other diseases.
Methylene blue should be discarded if your thyroid gland is destroyed or removed. The product is incompatible with silver water, thyroxine and potassium permanganate. Any chemical drugs in combination with iodinol can give an unexpected reaction, so first consult your doctor.
It is a pity that we are gradually weaned from such simple medicines. Well, still, if there would be a worthy alternative, and even with any symptoms, we are almost immediately stuffed with antibiotics. Then we wonder why we have such a weak immune system.
Tell your friends about blue iodine so that they also remember the forgotten remedy!
An experienced livestock breeder, besides the usual medicines, always has an antiseptic on hand. If attentive parents buy green for children, then wise farmers for birds buy blue. Methylene blue for chickens is a cheap preparation that can surprise with a wide spectrum of action and will help to cope with many diseases.
Bacteria were the first inhabitants of the planet Earth. They live literally in every corner of the planet, even in the organisms of living beings. For example, in the human body is more than 2 kg of bacteria, which are mostly harmless. However, sometimes pathogenic microbes come to visit and begin to adversely affect the work of the cells. An effective means of combating these pests are antiseptics.
A few centuries ago, the concept of disinfection did not overlap with medicine, until the Austrian Ignaz Semmelweis did not find a connection between dirty hands and an increase in mortality. With his “clean” hand, the Scotsman Joseph Lister invented the first antiseptic in the 19th century.
These disinfectants are actively used to heal wounds, and to eliminate bacilli and fungal infections.
There are several groups of antiseptics.
| Group | Representatives |
|---|---|
| Halogenated | chlorine, iodine |
| Oxidizing agents | hydrogen peroxide, potassium permanganate |
| Metal compounds | preparations of bismuth, zinc, lead |
| Acids and alkalis | salicylic and boric acids, sodium tetraborate, benzoyl peroxide |
| Aldehydes | cidipol |
| Alcohols | ethanol |
| Phenols | resorcinol |
| Anionic | soap |
| Herbal preparations | marigold or chamomile flowers |
| Colorants | methylene blue, brilliant green |
Many of them have flaws that appear when used externally, such as chlorine, the pungent smell of which irritates the mucous membranes. Methylene blue, on the contrary, does not adversely affect mucous membranes.

The popularity of dyes as disinfectants: green, red or blue
It's amazing how many coloring substances turned out to be antiseptics. Take, for example, methylene blue: traditionally it was used to "convert" tissues in blue. However, he was prepared for a more significant health mission.
Comparative characteristics of the pharmacological properties of antiseptic dyes
| Name | Indications for use | Release form |
|---|---|---|
| Brilliant green |
| Available in the form of alcohol solutions of green (1% and 2%), as well as in the form of pencils. |
| Magenta |
| Aqueous solutions are obtained in a saturated red color, but the drug is not used on its own, only as part of some combined antiseptics, in particular, fucorcin. |
| Methylene blue |
| Available in powder and in ampoules containing 20 and 50 ml each of 1% solution of methylene blue in 25% glucose solution. |
As can be seen from the table, the narrowest spectrum of action of Zelenka, moreover, has recently been actively debated about its effectiveness as an antiseptic. The greatest radius of impact is blue, the disinfectant properties of which are successfully used in the livestock sector.
Medicines - an important component of the arsenal of any poultry farmer. We understand what sets of first-aid kits exist, what preparations are used and what they are used for, as well as how to assemble a first-aid kit for chickens with their own hands.
Composition and release form
Methylene blue is included in the category of antiseptics. In veterinary medicine it is widely used for the prevention and treatment of the following diseases:
- fungal fish diseases;
- rheumatic diseases of horses;
- infectious diarrhea of sheep and goats.
He also showed excellent performance in the treatment of diseases of birds caused by various infections.

Characteristics of the drug
You can buy at any pharmacy, it is released without a prescription.

| Appearance | Crystal granules |
|---|---|
| Solubility | In water it is diluted in a proportion of 1:30, slightly soluble in alcohol. |
| Storage conditions | In a tightly closed container, in a dark place at a temperature of from 150 ° C to 250 ° C. |
| Release form | Powder and ampoules containing 20 and 50 ml of 1% solution of methylene blue in 25% glucose solution each; alcohol solution: in glass bottles of 10 ml. |
| Shelf life |
|
| Composition | 10 ml of alcohol solution:
|
The principle of blue action is the ability of the active substance to form compounds with the proteins of the pest cell, which contributes to the destruction of harmful bacteria.

Use of methylene blue for treating chickens
Traditionally, this tool is used to disinfect the premises, however, the range of its use is not limited to this:
On damaged tissues, methylene blue creates a protective layer.
Methylene blue can not be called a panacea, curing 100% of all possible ailments, but in some diseases it can become a great helper, especially in combination with other means.
Mode of application
The preparation is intended both for external, and for internal use.
When treating the skin with a preparation, the injured areas and the adjacent healthy areas are treated. Avoid contact of fluid with mucous membranes.
Drug dosage
Depending on the type of disease and the method of use, the permissible amount of the drug is changed.
Allowable concentrations of methylene blue
| Disease | Permissible concentration |
|---|---|
| Skin lesions | Wash wounds with 1 - 3% solution on alcohol. |
| Bursitis | To enter into wounds 2% solution at the rate of 0.01% per 1 kg of chicken weight. |
| Infectious diseases of the urinary tract | Rinse with 0.02% aqueous solution. |
| Infectious GIT | Give with a liquid in a ratio of 1: 5000. |
| Poison poisoning | Give intravenously 0.1-0.25 ml of 1% solution per 1 kg of chicken weight. |
| Poisoning with cyanide, hydrocyanic acid or hydrogen sulfide | Give intravenously at the rate of 0.5 ml of 1% solution per 1 kg of chicken weight. |
As can be seen from the table, the main directions of use of the drug: the fight against infections and poisoning, as well as treatment of wounds.

Side effects
The drug has practically no contraindications, with the exception of individual intolerance, manifested in the appearance of allergic skin reactions. It is not recommended to apply the solution to the mucous membranes.

Symptoms of diseases of chickens and the rules of their treatment
Most avian diseases are caused either by illiterate care and nutrition, or by the presence of infections that are often not treatable.


Infectious diseases of chickens and their treatment
Unfortunately, the environment contains many viruses and microbes that provoke diseases of birds. If the bird's body is weakened, it will be a tasty morsel for the growth of harmful bacteria and microbes, which, when released, begin to multiply at great speed. The main danger lies in the fact that a single flock can infect an entire herd and provoke a 100% lethal outcome.


There are several types of contagious diseases:
- infectious;
- fungal;
- helminth and diseases caused by the harmful effects of insects.
Infectious diseases
The most common are the following diseases:
- bronchitis;
- pasteurellosis;
- coccidiosis;
- colibacteriosis;
- pullorosis;
- smallpox;
- salmonellosis;
- mycoplasmosis;
- bursitis.
Frequently encountered Marek's disease, avian flu and Newcastle disease are not treatable.
See also: Newcastle disease
Newcastle disease is among the incurable diseases of chickens. What to do so that the birds do not get sick? Vaccination and prevention of Newcastle disease, quarantine.
Infectious Disease Treatment Scheme
| Disease | Symptoms | Treatment |
|---|---|---|
| Pullorosis |
| Antibiotic injections as prescribed by the veterinarian and obligatory disinfection of the chicken coop, the use of methylene blue is possible. |
| Salmonellosis |
| Enrofloxacin, neomycin, tetracycline, gentamicin, furazolidone or streptomycin, antibiotic injections, water with a small amount of potassium permanganate or methylene blue are prescribed. |
| Infectious bursitis |
| In the bursa pour 2% blue solution. |
| Infectious diseases of the digestive tract |
| Blueprint is given with drinking in the proportion of 1: 5000. |
| Streptococcosis |
| Prescribe a course of antibiotics and disinfection measures using methylene blue. |
| Smallpox |
| In addition to taking antibiotics, skin patches should be treated with a solution of furatsilina or methylene blue. |
| Ornithosis |
| Assign a course of antibiotics, fortified food and drink with the addition of potassium permanganate and methylene blue in a ratio of 1: 5000 alternately. |
| Omphalite |
| Antibiotics, the abdomen should be lubricated with a solution of methylene blue. |
| Coccidiosis |
| A course of antibiotics, fish oil should be added to the food, and a small amount of methylene blue should be added to the drink. |
| Prescribe a course of antibiotics and drink with a small amount of potassium permanganate or methylene blue. | |
| Hemophilus or runny nose |
| Drinking water should be diluted with a small amount of disinfectant, it is possible to use methylene blue. |
| Bronchitis |
| Aerosol treatment of the chicken coop in the presence of poultry using antiseptics: iodine monochloride, monklavit, ASD-2, ecocide, or methylene blue. |
| Colibacteriosis |
| Enrofloxacin is prescribed. In food add furazolidone at the rate of 4 g per 1 kg of the mixture, and in the water a small amount of methylene blue. |
| Mycoplasmosis |
| Assign a choice: farmazin at the rate of 1 g per 1 l, tylosin or tylan - 0.5 g per 1 l, tilmicovet - 3 ml per 1 l, pneumotyl - 0.3 ml per 1 l, enroflox 10%, enroxil 10% or enroflon 10% - 1 ml per liter. Add a small amount of methylene blue to the water. |
| Pasteurellosis |
| Assign chloramphenicol with food 2 to 3 times a day at the rate of 60 -80 mg per 1 kg of body weight; tetracycline, doxycycline, oxytetracycline: 50–60 mg per 1 kg of weight; norsulfazol: 0.5 g twice a day; Spectam: 1 g per 1 liter of water; avelox: 1 g per liter or 2 g per 1 kg of feed; Floron: 1 - 2 ml per 1 l; spelink: 1.1 g per 1 kg of mass; Add a small amount of methylene blue to the water. |
Methylene blue is practically not used as a monopreparation, but in combination with other drugs it is an effective aid in the fight against ailments.

Fungal diseases
Compared with infectious, fungal diseases are not so terrible. However, they should not be ignored, since they are also infectious and spread very quickly. Most of them are provoked by the lack of proper cleaning of the chicken coop.


Treatment of fungal diseases
Unlike aspergillosis, ringworm is not treated.
Helminthic diseases and diseases caused by the harmful effects of insects
Treatment regimen for some helminthic diseases
| Disease | Symptoms | Treatment |
|---|---|---|
| Ascariasis |
| Assign a course of hygromycin B, carbon tetrachloride and phenothiazine, methylene blue is added to water in a ratio of 1: 5000. |
| Drepanidoteniasis |
| Assign fenasal and mikrosal. From popular methods it is recommended to give garlic and pumpkin seeds. In drinking should be added methylene blue in the proportion of 1: 5000. |
| Pliers |
| Treatment of birds with any permitted insecticides: Sevin, pyrethrum or ecophils - one bird is not more than 15 g. Powder should be sprayed on the surface of feathers, and diluted using an aerosol to disinfect the chicken coop. Methylene blue can be used as a disinfection aid. |
Bedbugs, lice, worms and fleas,of course, not as dangerous as infectious diseases, but in some cases, if you do not start timely treatment, they can be fatal.
federal State Autonomous Educational Institution of Higher Professional Education
"BELGOROD STATE NATIONAL RESEARCH UNIVERSITY"
(National Research University "BelSU")
BIOLOGICAL AND CHEMICAL FACULTY
DEPARTMENT OF COMMON CHEMISTRY
METHYLENE BLUE
Course work
full-time female students of the 3rd year group 041004
Ushakova Natalia Sergeevna
Supervisor:
phD in Chemistry,
senior teacher Simakov SV
BELGOROD 2013
Introduction
Literature review
1 Methylene Blue Chemistry
2Preparation of methylene blue
2.1 Synthesis of methylene blue by Karo
2.2 Synthesis of methylene blue according to Berntsen
2.3 Synthesis of methylene blue according to Kerman
3Application of methylene blue
experimental part
1 Selecting the optimal wavelength
2Production of calibration curve
Conclusion
Bibliography
Introduction
<#"justify">1.Literature review
1.1Methylene Blue Chemistry
Methylene blue (MG) was first synthesized by the German chemist Heinrich Karo in 1876, who also first (together with Bayer) obtained synthetic indigo, and also discovered peroxomonosulfuric acid, named after the famous chemist Karo.
Methylene blue (lat. Methylenum coeruleum) (N, N, N`, N "-tetramethylthionine chloride trihydrate, 3,7-chloride, methyl blue, methylene blue). They are dark green crystals with a bronze sheen, dissolved in water by staining the solution in dark blue.
The chemical formula is C16H18CIN3S or as crystalline hydrate C16H18CIN3S · H2O.
The molecular weight is 319.86 g / mol. Solubility: slightly soluble in water, ethanol, practically insoluble in diethyl ether and chloroform. E0 = + 0.53 V is easily restored. In aqueous solutions, the monomeric form absorbs light in ? max = 668 nm, dimer - in ? max = 612 nm; the dimerization constant Kdim = 5.00 · 103 (pH 4-5.5).
1.2Methylene blue production
Methylene blue - tetramethyl substitution of thionin, occupies a prominent place among the dyes.
1.2.1Synthesis of methylene blue by Karo
Synthesis of methylene blue was first carried out by Caro (1876), its structure clarified by Beritsen.
The first synthesis of methylene blue was carried out by the reaction of Lauta and consisted in the oxidation of asym-dimethyl-n-phenylenediamine and hydrogen sulfide with ferric chloride. At the same time, as an intermediate product, indamium salt (Brindshedler green) is formed, which then, condensing with hydrogen sulfide, turns into a thiazine dye:
As well as for the dyes of the oxazine series, for dyes of the type methylene blue are now considered to be equal and ortho-and para-quinoid formulas.
2.2Synthesis of methylene blue according to Berntsen
The old synthesis of methylene blue by Caro was subsequently replaced by synthesis by Berntsen, based on 1 mol. dimethyl-n-phenylenediamine, 1 mol. dimethianiline and sodium thiosulfate.
The initial product in the synthesis of methylene blue is nitrosodimethylaniline, which is reduced by zinc dust in hydrochloric acid in n-aminodimethylaniline (I). Under the action of thiosulfate in a neutral medium in the presence of sodium bichromate and zinc chloride, n-aminodimethylaniline is converted into dimethyl p-phenylene diamine thiosulfonic acid (II).
Dimethyl-n-phenylenediamine thiosulfonic acid is reacted with dimethylaniline in the presence of sodium dichromate in an acidic medium. This forms a sparingly soluble tetramethiindamin thiosulfonic acid (III):
Tetrametiindine thiosulfonic acid, when heated with zinc chloride in the presence of hydrochloric acid, detaches sulfurous acid, turning into a dye M.G
In the production of methylene blue, the dye is usually isolated as a double salt with zinc chloride, called methylene blue C:
Zinc is precipitated with soda solution, and the dye is obtained in the form of chloride.
1.2.3Kerman synthesis
Finally, the third methylene blue synthesis proposed by Kerman should also be mentioned. This synthesis does not have industrial value, but deserves attention because it establishes a simple genetic connection between the ancestor of compounds of this class, thiodiphenylamine, and the dye. Under the action of excess bromine on thiodiphenylamine, phenazthionium perbromide is formed, which, combining with dimethylamine, is converted into methylene blue. This process apparently proceeds in two stages: first, the amine is attached to the quinoid ring, and then intramolecular oxidation, with the Br3 group playing the role of oxidizer.
1.3Use of methylene blue
Methylene blue is used for the manufacture of ink and pencils and for coloring paper, can be used in dyeing cotton on tannin mordant, as well as silk. Due to the fragility of the formed colors, its application in the technical industry is limited.
Methylene blue is the first synthetic drug used in medical practice. This drug was used to treat malaria in the early 20th century, but during the Pacific War of 1937-1945 it was rejected by the Allied forces: you see, writing with a blue jet turned out to be non-comic. Now in medicine<#"101" src="doc_zip10.jpg" />
Methylene blue reduction reaction:
There were difficulties in determining the redox potentials of methylene blue. Methylene blue was poorly cleanable; when dried above 110 ° C it decomposed. Analysis of carefully recrystallized samples showed a high content in them and, therefore, the presence of electroactive impurities. To eliminate this interference, we had to extract a number of trade preparations and compare all the results obtained for each of the samples. It has been established that the redox potential of methylene blue at pH = 7 is + 0.011 V, and at pH = 0 + 0.53 V. The change in its color occurs when the redox potential changes from + 0.54 to –0.146 V. The main advantage of methylene blue is its intense color (much brighter than, for example, the color of indigosulfonates), and therefore it can be used in dilute solutions. In addition, it is in the usual potential region, where the choice is not rich.
b. Metallochromic indicator<#"justify">MetallurBeddingNote NoteMg10 colorless Ammonia buffer Ca10 colorless Ammonium buffer Cd10 colorless Ammonium buffer Co (II) 10 colorless Ammonia buffer Ni10 colorless Ammonia buffer Zn10 colorless Ammonium buffer
The table shows the metals that can be directly titrated complexometric in the presence of this indicator. Metals that are determined by indirect methods or by the back titration method are not indicated. Each indicator can be used for this type of titration.
with. Acid-based indicator
Methylene blue has the properties of acid-base indicators.
A clear color change is shown by those indicators, the acidic and basic forms of which are colored in additional colors, for example red and green. There are no such ideal indicators, therefore, by adding an inert dye both indicator colors change so that they become additional colors. Instead of turning the red color to yellow, characteristic of methylene orange, methylene red and dimethyl yellow, you can add a blue dye, such as methylene blue, to get a more contrast transition from purple to green. The color change of such a mixed indicator, consisting of a mixture of methylene blue and other indicators, is shown in Table 2.
Table 2. Mixed Indicators
The table shows the titration index (pT) - the pH value at which the color change of the indicator is clearly noticeable.
For the determination of mercury cations in the form of methylene blue polyiodide:
For which, an excess of a solution of iodine in potassium iodide is added to a solution of methylene blue. When standing in the cold, a water-insoluble polyiodide precipitates as a precipitate of almost black color. When a solution of a salt of divalent mercury is added to an aqueous suspension of polyiodide, iodine associated with the dye is converted into a low-dissociated salt with mercury. In this case, the dye is released, giving the solution a blue color. The reaction is very sensitive.
As a sensitive reagent for the perchloric acid anion СlO4-, in the presence of which red-violet staining is formed.
Of the substituted methylene blue, its nitro derivative, obtained by nitrosing the dye and subsequent oxidation of the nitroso group with dilute nitric acid, is interesting. This dye is known as methylene green B
The combined oxidation of dimethyl-n-phenylendiimine-thiosulfonic acid with methylethylaniline leads to the formation of basic blue (I).
Stains with a more reddish tinge than those treated with methylene blue give novomethylene blue (II) obtained from aminoethyl-o-toluidine in the same way as methylene blue
Asymmetric dimethylthionine - azure I is obtained by oxidation of methylene blue bichromate, accompanied by partial demethylation. Azur I is used in histology:
methylene blue indicator kerman
Methylene blue as an agent for determining the specific surface of porous materials:
Methylene blue is used to determine the adsorption capacity of activated carbons.<#"15" src="doc_zip18.jpg" />H + + Cl-
Consider the mechanism of the process of adsorption of methylene blue on the surface of porous materials:
The mechanism of sorption is a very complex issue and we can’t say for sure, but from what is known from the literature, we can speak about the ion-exchange mechanism of sorption. The adsorption of the dye methylene blue on the surface of porous materials is ion-exchange adsorption, that is, there is a mutual exchange of ions between the solution of methylene blue and the solid surface. When this occurs, the adsorption of ions from a solution of methylene blue and the desorption of ions from a solid surface into a solution. The MG molecule can exchange not for one calcium ion, but for two or more ions.
For the quantitative description of adsorption, mainly two quantities are used. One is measured by the amount or mass of the adsorbate, that is, the number of moles or grams per unit of surface area or unit of mass of the adsorbent (for a solid in a powdery state); this value is usually denoted by the letter A.
Another characteristic of the amount of adsorption is determined by the excess of the substance in the surface layer of a certain thickness compared to its quantity in the same phase volume, also referred to as a unit of surface area or a unit mass of the adsorbent. This value is called Gibbs adsorption and denoted by the letter G.
If an adsorbent of mass m is placed in a solution of volume V with a first initial concentration of C0, then part of the substance from the solution will be adsorbed on a solid surface. As a result, the concentration of the substance will decrease with becoming equal to C. A decrease in concentration by the value of C0 - C occurred as a result of the adsorption of the substance on the surface of the adsorbent, the amount of excess adsorption G (mmol / g) can be determined by the formula:
where C0 is the initial concentration of the adsorbate, mol / l;
C is the equilibrium concentration of the adsorbate, mol / l;
V is the volume of solution, ml;
m is the mass of the sorbent,
Determination of the specific surface area according to the BET method includes two stages: estimation of the capacity of the monolayer G by the adsorption isotherm and calculation according to Gvelichina Sud using the molecular area w0. The area of one methylene blue cation, according to X-ray structural analysis, is 95.6 Å2. If the maximum sorbed amount of methylene blue corresponds to a monomolecular dye layer on the sorbent surface, then the value of the specific surface can be calculated by the formula (2):
beats = r? * NA * w0 (2)
Where is the magnitude of the limiting sorption? determined from the graph by the tangent of the angle:
NA is the Avogadro constant, numerically equal to 6.06.1023 mol-1; w0 is the area occupied by the same methylene blue cations, m2.
2.experimental part
Materials and equipment: 0.001 M solution of methylene blue, 25 ml volumetric flasks, 1 ml, 5 ml pipettes, 10 ml; glass glasses with a capacity of 150 ml; cuvettes 10 mm thick spectrophotometer Specord 50.
The course of the analysis:
2.1Selection of the optimal absorption wavelength
Place a solution of methylene blue (C = 0.1 g / mol) in a cuvette with a thickness of 10 mm, measure the optical density on a spectrophotometer at various wavelengths. Distilled water is used as a reference solution. The results are recorded in the table and build the dependence of the optical density of this solution from different wavelengths.
Fig.1. The dependence of the optical density of a solution of methylene blue on various wavelengths.
In accordance with the data obtained, the optimal absorption wavelength is 665 nm.
2.2Construction of the calibration curve
In seven numbered 50 ml volumetric flasks, 1,2,4,5,7,10,50,5 ml of a standard solution of 1 * 10-3 M methylene blue is placed and adjusted to the mark with distilled water, the optical density is measured on a spectrophotometer at 665 nm using cuvettes 10 mm. Distilled water was used as a comparison solution.
Fig. 2. The dependence of optical density on the concentration of the solution of methylene blue.
Conclusion
Methylene blue is a dark green crystals with a bronze sheen, was first synthesized by Heinrich Caro<#"15" src="doc_zip25.jpg" />H + + Cl-
Bibliography
1.# "justify"\u003e 2. The course of organic chemistry. Translated from the German 13th revised and updated edition. V.E. Wasserberg, E.M. Levina and L. D. Rodionova. Edited by M. N. Kolosov. L., -1960. - 1216 s.
.I.M. Kogan. Chemistry dyes. Third Edition. Edited by prof. A.I. Koroleva.M., 1956. -696 p.
.Indicators: in 2 volumes. Volume 2. Translated from English by I. V. Matveyeva. Edited by Dr. Chem. Sciences I. N. Marova. Publisher Mir, M., 1976. - 446 p.
.Indicators: in 2 volumes. Volume 1. Translated from English by I. V. Matveyeva. Edited by Dr. Chem. Sciences I. N. Marova. Publisher Mir, M., 1976. - 496 p.
.Rabinovich V. A., Havin Z. Ya. Brief Chemical Reference: Ref. Ed. / Ed. A. A. Potekhin and A. I. Efimov. - 3rd ed., Pererab. And dol. - L .: Chemistry, 1991.- 432 p.
.GOST 4453 - 74. Active lightening charcoal powdered charcoal.
.Voyutsky S.S. The course of colloid chemistry. - M .: Chemistry, 1975. - 512 p.
.Greg S., Singh K. Adsorption, specific surface area, porosity. - M .: Mir, 1970. - 408 p.
Work order
Our experts will help to write a work with a mandatory check for uniqueness in the Antiplagiat system.
Send an application with the requirements right now to find out the cost and the possibility of writing.
Analogs
These are drugs belonging to the same pharmaceutical group, which contain different active substances (INN), differ in name from each other, but are used to treat the same diseases.
- - Aerosol for inhalation
- - Solution for injection 0.4 mg / ml
- - Substance-powder 0.5,1,5,10,35 and 40 kg
- - Solution for intravenous administration
- - Solution for intramuscular and subcutaneous administration
- - Substance-powder 0.5 kg; 1 kg
Indications for use of the drug Methylene blue alcohol solution 1%
Burns, pyoderma, folliculitis; infectious and inflammatory diseases of the urinary tract, including cystitis, urethritis; poisoning with cyanides, carbon monoxide, hydrogen sulfide, methemoglobin-forming poisons (nitrites, aniline and its derivatives), drug methemoglobinemia
The release form of the drug Methylene blue solution alcohol 1%
alcohol solution 1%; dropper bottle 10 ml;
Pharmacodynamics of the drug Methylene blue alcohol solution 1%
Is an acceptor or donor of protons in redox reactions. The antiseptic effect is due to the interaction with mucopolysaccharides and proteins of microorganisms. In low concentrations restores methemoglobin to hemoglobin, in high concentrations - on the contrary, it oxidizes hemoglobin. It has antidote properties in relation to aniline, potassium permanganate, hydrocyanic acid. Action with hydrocyanic acid poisoning is based on the ability (in large doses) to translate hemoglobin into methemoglobin, which binds to cyanide, forming a non-toxic cyanmethemoglobin.
Pharmacokinetics of the drug Methylene blue alcohol solution 1%
After the on / in the introduction of approximately evenly distributed in the body. Excreted in the urine mostly unchanged and in small quantities as a metabolite, leukemethylene blue. It paints urine blue, and therefore can be used to study the functional ability of the kidneys.
Contraindications to the use of the drug Methylene blue alcohol solution 1%
Hypersensitivity.
Side effects of the drug Methylene blue solution alcohol 1%
Allergic reactions; systemic effects: nausea, vomiting, abdominal pain, pain in the kidneys and bladder, headache, loss of appetite, mental discomfort.
Dosage and administration of the drug Methylene blue alcohol solution 1%
In / in, externally. As an antiseptic: for skin diseases - externally 1% alcohol solution; with cystitis and urethritis - washing the cavities with an aqueous solution of 1: 5000 (0.02%). As an antidote: in case of poisoning with cyanide, carbon monoxide, hydrogen sulfide - in / in, very slowly, 50-100 ml of 1% aqueous solution; in case of poisoning with methemoglobin-forming poisons - in / in, in small doses (0.1–0.15 ml of 1% aqueous solution per 1 kg of body weight).
Precautions when taking the drug Methylene blue solution alcohol 1%
With care prescribed parenterally to women of childbearing age. Do not enter the SC, intrathecally. Introduce in / in should only be very slow (with interruptions for a few minutes).
Storage conditions of the drug Methylene blue alcohol solution 1%
B. List: In the dark place, in a tightly closed package, at a temperature not exceeding 30 ° C.
The shelf life of the drug Methylene blue alcohol solution 1%
Affiliation of the drug Methylene blue alcohol solution 1% to ATX-classification:
V Other drugs
V03 Other miscellaneous drugs
V03A Other miscellaneous drugs
All well-known, effective and affordable drug for the treatment of a number of diseases can be called Methylene Blue. Its antibacterial, analgesic and antidote properties are used in many areas of medicine.
In superficial use, methylene blue is used for burn lesions, purulent and inflammatory processes of the skin.
Analogs
In the pharmacy you can find modern analogues of the drug, where the active ingredient remains the same, just the name, the percentage and additional substances are different:
- methylthioninium chloride;
- methylene blue and the name of blue methylene;
- methyl blue;
- methylene bleu.
In other cases, the only drug close to methylene is brilliant green (brilliant green).
Application
Blueprint is used in many areas of medicine: therapeutic and surgical dentistry, nephrology, toxicology, dermatology, general surgery.
But the first place in the activity of its use belongs to dentistry. It is recommended to resort to methylene blue when there are such diseases as: stomatitis, gingivitis, abscesses, furuncles and phlegmons in the mouth.

Methylene blue helps in the elimination of methemoglobinemia, felon, surface pyoderma, burns, intoxications caused by hydrogen sulphide, chadny gas, aniline, nitrate substances and hydrocyanic acid. In nephrology, it is used for the treatment of urethritis, cystitis and for the purpose of diagnosis, after ingestion, a solution of methylene blue gives the urine a bluish tint.
Pharmacodynamics
Methylthioninium chloride acts as an active component in the preparation; it exhibits a disinfecting, analgesic effect, participates in oxidation and reduction processes, serves as a transporter of hydrogen molecules.
The main characteristic of a medicinal substance is the ability to create poorly soluble complex compounds with mucopolysaccharides and the protein part of pathogenic microflora cells. This destroys microorganisms. The use of externally powdered methylene, eliminates its entry into the circulatory system.
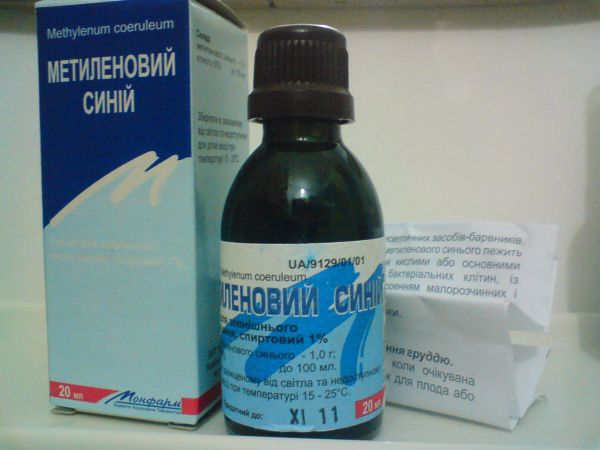
Available as:
- Powdered methylene 10 g.
- 1% alcohol solution (10 and 15 ml).
- 1% solution in 25% glucose (20 and 50 ml ampoule packs).
Instructions for use of methylene blue
Blue, like a powder, is an organic thiazine dye; it looks like dark greenish crystals with a bronze tint. They are slightly soluble in water and ethyl alcohol.
Chloroform and diethyl ether as solvents are also not suitable for it.
1st% alcoholic methylene is applied to damaged, pre-cleansed skin and healthy areas around the site of injury.
0.02% aqueous solution (the powder is diluted in water, a ratio of 1: 5000) is used for washing with cystitis and urethritis.
0.1 g of methylene blue is taken orally 3-4 times a day. Depending on the age for children, the dosage is calculated on the basis of 0.005-0.01 g for each year of life.
The 1st aqueous or 1% glucose solutions prepared on the 25th glucose are administered intravenously in case of poisoning with nitrites, anilines, hydrocyanic acid, carbon monoxide and other substances that form methemoglobin cores. Medical blue is introduced at the rate of 0.1-0.15 ml per kilogram of human weight.
What is methylene blue? It is an aniline dye and is used not only in medicine. But as an antidote, blueprint function in toxicology and hematology is to restore methemoglobin to hemoglobin. Overdosing leads to the reverse process - hemoglobin is oxidized to methemoglobin, a state where oxygen in the tissues and organs cannot flow with blood, which inhibits their metabolic processes.
Side effects
Superficial blue can cause a local allergic reaction, especially with large areas of damage.
Introduced quickly intravenously drug or its overdose in some cases cause:
- vomiting, nausea, lack of appetite, pain in the epigastric region;
- urinary tract and kidney area;
- pain in urea
- headache;
- mental disorder;
- symptoms of anemia.
Use in dentistry

For stomatitis aphthous, aphthous and intact tissues around are dotted with a methylene blue one-percent solution on water applied to a cotton swab or cotton-gauze tampon every two or two and a half hours. The drug can be combined with other drugs. The treated aphthae are lubricated with stotidine, and after an hour, the mouth cavity is rinsed and Shostakovsky balsam is applied.
1-2% methylthioninium chloride aqueous solution is used to treat gingivitis. Methyl blue smear the affected areas of the gums. The frequency of repetition of procedures depends on the extent of infection, but not less than three times a day.
The composition of methylene blue is allowed in the conditions of modern medicine to successfully fight candidal stomatitis.
1% methylene blue is applied to the places of spread of the fungus: for adults - from 6 to 15 times, for children - from 3 to 6. If everything is done correctly, the disease and its symptoms will begin to fade on the fourth day.
Contraindications
Blue should not be used with individual intolerance to the components of the drug (methyl, thiazine dye, ethanol, glucose). The drug is not shown to children under one year.
Pharmacy blue is sold ready to use, the main thing is to know what kind of medicine (in percentage concentration) is based on it.
Shelf life of the drug - up to two years.
Methylene blue price
| A country | City | Price |
|---|---|---|
| Russia | Moscow | 33 - 40 rubles |
| Russia | St. Petersburg | 32-39 rubles |
| Russia | Kazan | 33 - 38 rubles |
| Russia | Yekaterinburg | 32 - 39 rubles |
| Ukraine | Kiev | 12 - 23 UAH. |
| Ukraine | Odessa | 13 - 24 UAH |
| Ukraine | Kharkov | 12 - 23 UAH. |
A prescription for the purchase of medicines is not required.
Video




