Reactions with non-metals. General characteristics of non-metals
Allotropy
From the \\ (118 \\) currently known chemical elements \\ (22 \\) of the element form simple substances with non-metallic properties. Non-metallic simple substances are much larger than the non-metallic chemical elements themselves. The reason for this is the existence of a phenomenon called allotropy.
Allotropy is the ability of atoms of a given chemical element to form several simple substances called allotropic modifications or allotropic modifications.
for example, the chemical element oxygen \\ (O \\) forms a simple substance oxygen O 2, the molecule of which consists of two atoms, and simple substance ozone O 3, the molecule of which consists of three atoms of the given element.
The chemical element phosphorus (P \\) forms many allotropic modifications, the most important of which are red phosphorus and white phosphorus.
The chemical element carbon \\ (C \\) forms naturally occurring modifications - diamond and graphite.
Allotropic modifications formed by the same chemical element differ significantly from each other both in structure and in properties.
Allotropy is not inherent in all non-metallic chemical elements.
for example, hydrogen, nitrogen, elements of the VIIA and VIIIA groups do not have allotropic modifications, i.e. Each of these elements forms only one simple substance.
Crystal lattice of non-metals
The reason for the large variety of physical properties of non-metals lies in the different structure of the crystal lattices of these substances.
Part of non-metals has atomic crystal lattice. Crystals of such substances are composed of atoms connected to each other by strong covalent bonds. Such non-metals are in a solid state of aggregation and are non-volatile. Examples of such substances are diamond, graphite, red phosphorus and silicon.
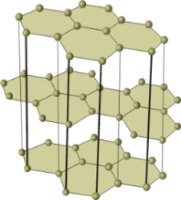
Crystal lattice models of diamond (left) and graphite. The crystals of these allotropic modifications consist of carbon atoms connected by covalent bonds. Graphite crystals, in contrast to diamond crystals, are composed of separate layers, which are arranged in relation to each other, just like sheets of paper in a book.
The other part of non-metals has molecular crystal lattice. In this case, in each molecule, the atoms are connected by a fairly strongly covalent bond, while the individual molecules are very weakly bound to each other in the crystals of the substance. Therefore, substances of molecular structure under normal conditions can be gases, liquids or low-melting solids.
Oxygen O 2, ozone O 3, nitrogen N 2, hydrogen H 2, fluorine F 2, chlorine Cl 2, bromine Br 2, iodine I 2, white phosphorus P 4, crystalline sulfur S 8 and inert gases are all substances, crystals which consist of individual molecules (and in the case of inert gases, of individual atoms as if playing the role of molecules).
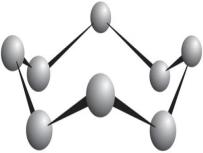

Sulfur molecule model (left) and sulfur crystal. A sulfur crystal is made up of individual molecules. \\ (S_8 \\).
Physical properties of non-metals
The properties of non-metallic simple substances are very diverse. As a matter of fact, they are united only by the fact that they, as a rule, do not possess those physical properties that are typical for metals, i.e. do not have the characteristic metallic luster, malleability, ductility, high thermal and electrical conductivity.
State of aggregation
Non-metals under normal conditions can be gaseous, liquid and solid substances.
Gaseous non-metals ihelium \\ (He \\), neon \\ (Ne \\), argon \\ (Ar \\), krypton \\ (Kr \\), xenon \\ (Xe \\) and radon \\ (Rn \\) come about. They are called inert or noble gases. Each "molecule" of inert gas consists of only one atom.
Such chemical elements as hydrogen \\ (H \\), oxygen \\ (O \\), nitrogen \\ (N \\), chlorine \\ (Cl \\), fluorine \\ (F \\) form gaseous substances consisting of diatomic molecules, respectively - H 2, O 2, N 2, Cl 2, F 2.
From non-metallic simple substances under normal conditions liquid is only bromine, whose molecules are diatomic Br 2.
The remaining non-metallic chemical elements under normal conditions are in solid aggregative state. For example, the chemical element carbon forms such solid substances as diamond and graphite. The crystalline sulfur S 8, the red phosphorus and the white phosphorus P 4, and the crystalline iodine I 2 are solid.
Color and shine
Only some non-metals, unlike metals, have a luster. For example, crystalline iodine, silicon and graphite are not similar to other non-metals - they have a luster, somewhat resembling the luster of metals.
If the vast majority of metals are characterized by silver-gray or silver-white color, the color of non-metals is very diverse. White color has white phosphorus, red - red phosphorus, yellow - sulfur and fluorine, red-brown - liquid bromine, yellow green - chlorine violet iodine vapors have color, blue - liquid oxygen, gray - graphite and silicon. Colorless is a diamond, stains do not have also inert gases, nitrogen, oxygen and hydrogen.
| |
Red phosphorus | White phosphorus |
General characteristics of non-metals.
Non-metals - chemical elements that form simple bodies that do not possess properties characteristic of metals. The qualitative characteristic of non-metals is electronegativity.
Electronegativity - is the ability to polarize a chemical bond, to pull away to itself the common electronic pairs.
Non-metals include 22 elements.
The position of non-metallic elements in the periodic table of chemical elements
1st period | |||||||
2nd period | |||||||
3rd period | |||||||
4th period | |||||||
5th period | |||||||
6th period |
As can be seen from the table, non-metallic elements are mainly located in the upper right part of the periodic system.
The structure of atoms of non-metals
A characteristic feature of non-metals is the greater (compared to metals) number of electrons at the external energy level of their atoms. This determines their greater ability to add additional electrons and the manifestation of a higher oxidative activity than metals. Especially strong oxidizing properties, i.e., the ability to attach electrons, manifest non-metals that are in the 2nd and 3rd periods of the VI-VII groups. If we compare the arrangement of electrons by orbitals in atoms of fluorine, chlorine and other halogens, then we can judge their distinctive properties. The fluorine atom has no free orbitals. Therefore, fluorine atoms can show only the valence of I and the degree of oxidation - 1. The strongest oxidizing agent is fluorine. In the atoms of other halogens, for example, in the chlorine atom, at the same energy level there are free d-orbitals. Due to this, the evaporation of electrons can occur in three different ways. In the first case, chlorine can show the degree of oxidation +3 and form chlorous acid HClO 2, which correspond to salts - chlorites, for example, potassium chlorite KClO 2. In the second case, chlorine can form compounds in which the degree of chlorine oxidation is +5. Such compounds include chloric acid HClO 3 and its salts - chlorates, for example, potassium chlorate KClO 3 (bertolet salt). In the third case, chlorine exhibits an oxidation state of +7, for example, in perchloric acid perchloric acid HClO 4 and perchlorates (in potassium perchlorate, KClO 4).
The structure of molecules of non-metals. Physical properties of non-metals
In a gaseous state at room temperature are:
hydrogen - H 2;
nitrogen - N 2;
oxygen - O 2;
fluorine - F 2;
chlorine - CI 2.
And inert gases:
helium - He;
neon - Ne;
argon - Ar;
krypton - Kr;
xenon - Xe;
radon - Rn).
In the liquid - bromine - Br.
In solid:
carbon - C;
silicon - Si;
phosphorus - P;
arsenic - As;
selenium - Se;
tellurium - Te;
astatine - At.
The range of colors is much richer for non-metals: red - for phosphorus, brown - for bromine, yellow - for sulfur, yellow-green - for chlorine, violet - for iodine vapors, etc.
The most typical non-metals have a molecular structure, and less typical - non-molecular. This explains the difference in their properties.
The composition and properties of simple substances - non-metals
Non-metals form both monoatomic and diatomic molecules. TO monatomic non-metals include inert gases, practically not reacting even with the most active substances. Inert gases are located in the VIII group of the periodic system, and the chemical formulas of the corresponding simple substances are as follows: He, Ne, Ar, Kr, Xe and Rn.
Some non-metals form diatomic molecules. These are H 2, F 2, Cl 2, Br 2, Cl 2 (elements of Group VII of the periodic system), as well as oxygen O 2 and nitrogen N 2. Of triatomic molecules consists gas ozone (O 3). For substances of nonmetals that are in a solid state, it is rather difficult to make a chemical formula. The carbon atoms in graphite are bonded to each other in various ways. It is difficult to isolate a single molecule in these structures. When writing chemical formulas for such substances, as in the case with metals, the assumption is introduced that such substances consist only of atoms. At the same time, chemical formulas are written without indices: C, Si, S, etc. Such simple substances as ozone and oxygen have the same qualitative composition (both consist of the same element — oxygen), but differ in the number atoms in a molecule have different properties. So, oxygen has no odor, while ozone has a sharp odor that we sense during a thunderstorm. The properties of solid non-metals, graphite and diamond, which also have the same qualitative composition, but a different structure, differ sharply (brittle graphite, diamond solid). Thus, the properties of a substance are determined not only by its qualitative composition, but also by how many atoms are contained in a molecule of a substance and how they are interconnected. Non-metals in the form of simple bodies are in a solid or gaseous state (except for bromine - liquid). They do not have the physical properties inherent in metals. Solid non-metals do not have the luster characteristic of metals; they are usually brittle, they do not conduct electric current and heat badly (with the exception of graphite). Crystalline boron B (like crystalline silicon) has a very high melting point (2075 ° C) and high hardness. The electrical conductivity of boron with increasing temperature greatly increases, which makes it possible to widely apply it in semiconductor technology. The addition of boron to steel and to alloys of aluminum, copper, nickel, etc. improves their mechanical properties. Borides (boron compounds with some metals, for example, with titanium: TiB, TiB 2) are necessary in the manufacture of parts for jet engines, gas turbine blades. As can be seen from scheme 1, carbon - C, silicon - Si, boron - B have a similar structure and have some common properties. As simple substances, they are found in two modifications - crystalline and amorphous. The crystalline modifications of these elements are very solid, with high melting points. Crystalline silicon has semiconductor properties. All these elements form compounds with metals - carbides, silicides and borides (CaC 2, Al 4 C 3, Fe 3 C, Mg 2 Si, TiB, TiB 2). Some of them have greater hardness, such as Fe 3 C, TiB. Calcium carbide is used to produce acetylene.
Chemical properties of non-metals
In accordance with the numerical values \u200b\u200bof the relative electronegativities, the oxidative capacity of nonmetals increases in the following order: Si, B, H, P, C, S, I, N, Cl, O, F.
Non-metals as oxidizing agents
The oxidative properties of non-metals occur when they interact:
with metals: 2Na + Cl 2 \u003d 2NaCl;
with hydrogen: H 2 + F 2 \u003d 2HF;
with non-metals that have a lower electronegativity: 2P + 5S \u003d P 2 S 5;
with some complex substances: 4NH 3 + 5O 2 \u003d 4NO + 6H 2 O,
2FeCl 2 + Cl 2 \u003d 2 FeCl 3.
Non-metals as reducing agents
All non-metals (except fluorine) exhibit reducing properties when interacting with oxygen:
S + O 2 \u003d SO 2, 2H 2 + O 2 \u003d 2H 2 O.
Oxygen in combination with fluorine may also exhibit a positive degree of oxidation, that is, it may be a reducing agent. All other non-metals exhibit reducing properties. For example, chlorine does not combine directly with oxygen, but indirectly, its oxides (Cl 2 O, ClO 2, Cl 2 O 2) can be obtained, in which chlorine exhibits a positive degree of oxidation. Nitrogen at high temperature directly combines with oxygen and exhibits reducing properties. Sulfur reacts even more easily with oxygen.
Many non-metals exhibit reducing properties when interacting with complex substances:
ZnO + C \u003d Zn + CO, S + 6HNO 3 conc \u003d H 2 SO 4 + 6NO 2 + 2H 2 O.
There are also such reactions in which the same non-metal is both an oxidizing agent and a reducing agent:
Cl 2 + H 2 O \u003d HCl + HClO.
Fluorine is the most typical non-metal to which reducing properties are uncharacteristic, that is, the ability to release electrons in chemical reactions.
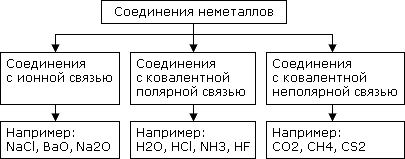
Non-metal compounds
Non-metals can form compounds with different intramolecular bonds.
Kinds of non-metal compounds
General formulas of hydrogen compounds by groups of the periodic system of chemical elements are given in the table:
Non-volatile hydrogen compounds | Volatile hydrogen compounds |
|||||
With metals, hydrogen forms (with some exceptions) non-volatile compounds, which are solids of non-molecular structure. Therefore, their melting points are relatively high. With non-metals, hydrogen forms volatile compounds of molecular structure (for example, hydrogen fluoride HF, hydrogen sulfide H 2 S, ammonia NH 3, methane CH 4). Under normal conditions, these are gases or volatile liquids. When dissolved in water, the hydrogen compounds of halogens, sulfur, selenium and tellurium form acids of the same formula as the hydrogen compounds themselves: HF, HCl, HBr, HI, H 2 S, H 2 Se, H 2 Te. When ammonia is dissolved in water, ammonia water is formed, usually denoted by the formula NH 4 OH and called ammonium hydroxide. It is also designated by the formula NH 3 ∙ H 2 O and is called ammonia hydrate.
With oxygen, non-metals form acid oxides. In some oxides, they exhibit a maximum oxidation rate equal to the group number (for example, SO 2, N 2 O 5), and others - lower (for example, SO 2, N 2 O 3). Acid oxides correspond to acids, and of the two oxygen acids of one non-metal, the one in which it exhibits a higher degree of oxidation is stronger. For example, nitric acid HNO 3 is stronger than nitrous HNO 2, and sulfuric acid H 2 SO 4 is stronger than sulfurous H 2 SO 3.
Characteristics of oxygen compounds of non-metals
The properties of higher oxides (i.e. oxides, which include an element of this group with the highest oxidation state) in the periods from left to right gradually change from basic to acidic.
In groups from top to bottom, the acidic properties of higher oxides gradually weaken. This can be judged by the properties of the acids corresponding to these oxides.
The increase in the acidic properties of the higher oxides of the corresponding elements in the periods from left to right is explained by the gradual increase in the positive charge of the ions of these elements.
In the main subgroups of the periodic table of chemical elements in the top-down direction, the acidic properties of higher oxides of non-metals decrease.
Halogens
The structure of halogen atoms
The halogens include elements of the VIII group of the periodic system, the atoms of these elements contain seven electrons at the external energy level, and before its completion they lack only one electron, therefore halogens exhibit bright oxidizing properties. In a subgroup, with an increase in the serial number, these properties decrease due to an increase in the radius of atoms: from fluorine to astatine - and, accordingly, their reducing properties increase. Similarly decreases the value of the relative electronegativity of halogens. As the most electronegative element, fluorine in compounds with other elements exhibits a constant degree of oxidation. -1 . The remaining halogens can exhibit both this oxidation state in compounds with metals, hydrogen and less electronegative elements, as well as positive odd oxidation states from +1 before +7 in compounds with more electronegative elements: oxygen, fluorine.
Simple substances halogens and their properties

Chlorine, bromine and iodine in glass vessels
Describing simple substances - halogens, it is necessary to recall the basic theoretical information about the types of chemical bonds and the crystalline structure of a substance. In diatomic halogen molecules, atoms are linked by a covalent non-polar bond G · · G or G ― G and have a molecular crystal lattice.
Under normal conditions F 2 - bright yellow, with an orange tint gas, Cl 2 - yellow-green poisonous gas with a characteristic suffocating odor, Br 2 - volatile brown liquid (bromine vapor is highly poisonous, bromine burns are very painful and do not heal for a long time), and I 2 - solid crystalline substance capable of sublimation. In row F 2, Сl 2 Br 2 I 2 - the density of simple substances increases, and the intensity of the color increases. Consequently, the change in the properties of atoms and simple substances, halogens, manifests the same regularity: with an increase in the serial number, the nonmetallic properties weaken, and the metallic properties increase.
Chemical properties of halogens
The interaction of halogens with metals with the formation of halides:
2Na + I 2 ―― 2Na +1 I -1 (sodium iodide);
2Al + 3I 2 \u003d 2Al +3 I 3 -1 (aluminum iodide);
2Al + 3Br 2 \u003d 2Al +3 Br 3 -1 (aluminum bromide).
During the reaction of metals of subgroups (transition metals) with halogens, halides are formed with a high degree of oxidation of the metal, for example:
2Fe + 3Cl 2 \u003d 2FeCl 3,
but 2HCl + Fe \u003d FeCl 2 + H 2.
The interaction of halogens with hydrogen to form hydrogen halides (the type of bond is covalent polar, the type of lattice is molecular). Comparison of the rate of chemical reactions of different halogens with hydrogen makes it possible to repeat its dependence on the nature of the reactants. So, fluorine has such a high reaction rate that it reacts with hydrogen with an explosion even in the dark. Under normal conditions, the reaction of chlorine with hydrogen proceeds slowly, and only when it is ignited or illuminated, does its speed increase many times (an explosion occurs). Bromine and iodine react even more slowly with hydrogen, and the latter reaction is already endothermic:
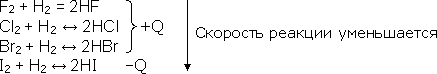
Only fluorine interacts with hydrogen irreversibly, the remaining halogens, depending on the conditions, can also give a reversible reaction.
Aqueous solutions of hydrogen halides are acids: HF — hydrogen fluoride (hydrofluoric), HCl — hydrochloric (hydrochloric), HBr — hydrobromic, HI — hydroiodic.
Halogens interact with water:
2F 2 + 2H 2 O \u003d 4HF + O 2
The water in the fluorine burns, oxygen is not the cause, but the result of burning, acting in the unusual role of a reducing agent.
To characterize the ability of some halogens (not halogen atoms, but simple substances) to displace others from solutions of their compounds, you can use the "activity series" of halogens, which is written as:
F 2\u003e Cl 2\u003e Br 2\u003e I 2,
i.e. oxidizing properties are reduced.
Thus, chlorine displaces bromine and iodine (but not fluorine), and bromine is able to displace only iodine from solutions of the corresponding salts:
2NaBr + Cl 2 \u003d 2NаСl + Br 2
2KI + Br 2 \u003d 2KBr + I 2.
Biological significance and use of halogens
Fluorineplays a very important role in the life of plants, animals and humans. Without fluoride, the development of the skeleton of the bone and especially the teeth is impossible. The content of fluoride in the bones is 80-100 mg per 100 g of dry matter. In enamel, fluorine is present in the form of the compound Ca 4 F 2 (PO 4) 2 and gives it hardness and whiteness. With a lack of fluoride in the human body, the tooth tissue is damaged (caries), and its excess contributes to dental disease with fluorosis. The daily human need for fluoride is 2-3 mg. Chlorine(chlorine-ion) is more important for the life of animals and humans than for plants. It is part of the kidneys, lungs, spleen, blood, saliva, cartilage, hair. Chlorine ions regulate the blood buffer system. Sodium chloride is an integral part of blood plasma and cerebrospinal fluid and is involved in the regulation of water metabolism in the body. Free hydrochloric acid is part of the gastric juice of all mammals and is actively involved in digestion. In a healthy person, 0.2-0.3% hydrochloric acid is contained in the stomach. A lack of chlorine in the body leads to tachycardia, lower blood pressure, seizures. A sufficient amount of chlorine is found in such vegetables as celery, radishes, cucumbers, white cabbage, dill, pepper, onions, artichokes. Brominealso included in the number of essential trace elements and most of it is contained in the pituitary gland, blood. Thyroid gland, adrenal glands. Bromides in small doses (0.1–0.3 adults) have a positive effect on the non-central nervous system as enhancers of the inhibition process in the cerebral cortex. In nature, bromides accumulate in plants such as rye, wheat, barley, potatoes, carrots, cherries, and apples. A lot of bromine is found in Dutch cheese. Iodine in the human body begins to accumulate in the womb. Human thyroid hormone thyroxin contains 60% of bound iodine. This hormone enters the liver, kidneys, mammary glands, and gastrointestinal tract with blood. The lack of iodine in the human body causes diseases such as endemic goiter and cretinism, in which growth slows down and mental retardation develops. In combination with other elements, iodine contributes to the growth and well-being of animals, improves their health and fertility. The main suppliers of iodine for humans are cereals, eggplants, beans, cabbage and cauliflower, potatoes, onions, carrots, cucumbers, pumpkin, lettuce, seaweed, squids.
State educational standardIntroduced since the approval of Moscow 2000. Overallcharacteristic directions of graduate training “Safety ..., types of interaction, alloys, application in engineering. Non-metals, properties, application, the most important compounds - oxides ...
Section 6 Education Content Primary General Education
DocumentIn nature. 3. Kingdom mushrooms (3 hours) Mushrooms. Overallcharacteristic mushrooms, their structure and livelihoods. Yeast ... recognition and production of substances. THEME 2 Non-metals (27 hrs.) Overallcharacteristicnon-metals: position in the periodic system DI ...
The main educational program of primary basic and secondary general education "secondary school number 10"
Basic educational programConcretized are common goals of the main in common education taking into account the specifics of the school subject; 2) commonfeature training ... in electrolyte solutions. Variety of substances Overallcharacteristicnon-metals based on their position in the periodic ...
All currently known chemical elements have a common "house" - the periodic system. However, they are not there as it should be, but in a strict order, a certain sequence. One of the main criteria by which all atoms are classified is the characteristics.
Non-metals and representatives of metal elements is the basis on which not only their separation within the table, but also the field of human use is based. Let's get acquainted closer with non-metals and their characteristics.
Position in the periodic system
If we consider the system of chemical elements as a whole, then we can determine the location of the position of non-metals as follows:
- Upper right corner.
- Above the conditional boundary diagonal from boron to astatine.
- The main subgroups with group IV-VIII.
Obviously, their number is clearly inferior to that of metals. By numerical ratio, it will be about 25/85. However, this fact does not diminish their significance and importance. At the same time, the physical properties of nonmetals are much more diverse than those of their "opponents".
Varieties of simple non-metal compounds
Define several main categories, which include all known considered elements. Physical properties - non-metals - allow you to separate them into:
- solid;
- gaseous;
- liquid.
At the same time there is a special group of elements - noble gases. According to their characteristics, they do not belong to any of the designated categories.

Gaseous non-metals
Those are quite a lot. These include such simple substances as:
- oxygen;
- nitrogen;
- halogens chlorine and fluorine;
- hydrogen;
- white phosphorus;
- ozone.
However, this is possible under the condition of standard environmental parameters. these representatives are molecular, the type of chemical bond in molecules is covalent non-polar. The physical properties of non-metals of this group are similar. They possess:
- compressibility;
- the ability of unlimited mixing between each other;
- extensibility;
- fill the entire volume of the vessel.
Among the substances listed are poisonous two - chlorine and very dangerous, asphyxiating compounds. At the same time, chlorine is a yellow-green gas, phosphorus is white, it is flammable in air.
Oxygen and ozone are good oxidizers. The first is a permanent component of air that is necessary for the life of most organisms. The second is formed after a thunderstorm under the action of electrical discharges of lightning on the oxygen of the air. It has a pleasant smell of freshness.

Liquid non-metals
The physical properties of the nonmetals of this group can be described by characterizing only one substance, bromine. Since only it is a liquid under normal conditions among all representatives of the considered group of elements.
It is a dark brown liquid, quite heavy, which is the strongest poison. Even bromine vapors can cause complex, non-healing ulcers on the hands. Its smell is very unpleasant, for which the element got its name (in translation bromos - offensive).

According to its chemical characteristics, bromine is an oxidizing agent for metals and a reducing agent for stronger non-metals than itself.
Despite such features, bromine ions must be present in the human body. Without it, there are diseases associated with hormonal disorders.
Solid representatives
Simple substances of this category include most non-metals. It:
- all carbon;
- red and black phosphorus;
- sulfur;
- silicon;
- arsenic;
- one of the modifications of tin.
They all have fairly hard but fragile substances. Black phosphorus is a dry compound, oily to the touch. Red is a pasty mass.

The hardest of all the designated substances is diamond - a type of carbon. The physical and chemical properties of non-metals of this group are very different, since some of them are located far from each other in the table. This means that the degree of oxidation, the chemical activity shown, the nature of the compounds — all these indicators will vary.
An interesting non-metal in the solid state is iodine. Its crystals glitter on the cut, thereby showing similarity with metals. This is not surprising, because it is located almost on the border with them. This substance also has a special property - sublimation. When heated, iodine enters a gaseous state, bypassing the liquid. Pairs of it have a bright purple rich color.
Physical properties of non-metals: table
To make it easier to identify what non-metals are, it is better to build a summary table. It will show what are the general physical properties of non-metals, and what are their differences.
| Physical property | Non-metal example |
| under normal conditions | All three are characteristic: solid (sulfur, carbon, silicon, and others), gaseous (for example, halogens), liquid (bromine) |
| Electrical and thermal conductivity | Not specific to anything other than carbon and black phosphorus. |
| Coloring simple matter | Very diverse. Example: bromine - red, sulfur - yellow, iodine crystals - dark violet, carbon in the form of graphite - dark gray, chlorine - yellow-green and so on |
| Metallic luster | Characteristic only for crystalline iodine. |
| Malleability and ductility | Completely absent. All solids are brittle, except diamond and some forms of silicon. |
It is obvious that the physical properties of non-metals are more dominated by differences than similarities. If there are several characteristics for metals that each of them will fall under, this is impossible for the elements we have considered.
If the majority of metal elements are not painted, the only exception is copper and gold, then almost all non-metals have their own color: fluorine is orange-yellow, chlorine is greenish-yellow, bromine is brick-red, iodine is purple, sulfur is yellow, phosphorus may be white, red and black, and liquid oxygen is blue.
All non-metals do not conduct heat and electric current, since they do not have free charge carriers - electrons, they are all used to form chemical bonds. Nonmetallic crystals are non-plastic and brittle, since any deformation leads to the destruction of chemical bonds. Most of the non-metals do not have a metallic sheen.
The physical properties of non-metals are diverse and are due to different types of crystal lattices.
1.4.1 Allotropy
Allotropy - the existence of chemical elements in two or more molecular or crystalline forms. For example, allotropes are ordinary oxygen O 2 and ozone O 3; in this case, allotropy is due to the formation of molecules with different numbers of atoms. Most often, allotropy is associated with the formation of crystals of various modifications. Carbon exists in two clearly distinct crystalline allotropic forms: in the form of diamond and graphite. Previously believed that the so-called. amorphous forms of carbon, charcoal and soot, are also allotropic modifications, but it turned out that they have the same crystal structure as graphite. Sulfur is found in two crystalline modifications: rhombic (a-S) and monoclinic (b-S); At least three of its non-crystalline forms are known: l-S, m-S and violet. White and red modifications are well studied for phosphorus, black phosphorus is also described; at temperatures below –77 ° C, there is another kind of white phosphorus. Allotropic modifications of As, Sn, Sb, Se were found, and at high temperatures - iron and many other elements.
1.5. Chemical properties of non-metals
Chemical elements-nonmetals can exhibit both oxidizing and reducing properties, depending on the chemical transformation in which they take part.
Atoms of the electronegative element itself - fluorine - are not capable of donating electrons, it always exhibits only oxidative properties, other elements can also show reducing properties, although much less so than metals. The most powerful oxidizing agents are fluorine, oxygen and chlorine, mainly reducing properties of hydrogen, boron, carbon, silicon, phosphorus, arsenic and tellurium. Intermediate redox properties are nitrogen, sulfur, iodine.
Interaction with simple substances
Interaction with metals:
2Na + Cl 2 \u003d 2NaCl,
6Li + N 2 \u003d 2Li 3 N,
2Ca + O 2 \u003d 2CaO
in these cases, non-metals exhibit oxidative properties; they accept electrons, forming negatively charged particles.
Interaction with other non-metals:
Interacting with hydrogen, most non-metals exhibit oxidative properties, forming volatile hydrogen compounds - covalent hydrides:
3H 2 + N 2 \u003d 2NH 3,
H 2 + Br 2 \u003d 2HBr;
Interacting with oxygen, all non-metals, except fluorine, exhibit reducing properties:
S + O 2 \u003d SO 2,
4P + 5O 2 \u003d 2P 2 O 5;
When interacting with fluorine, fluorine is an oxidizing agent, and oxygen is a reducing agent:
2F 2 + O 2 \u003d 2OF 2;
Non-metals interact with each other, the more electronegative metal plays the role of an oxidizing agent, the less electronegative - the role of a reducing agent:
S + 3F 2 \u003d SF 6,



