Chemical properties of organic substances in the schemes. Student's Guide to Organic Chemistry
SIBERIAN POLYTECHNICAL COLLEGE
STUDENT'S DIRECTORY
on ORGANIC CHEMISTRY
for specialties of technical and economic profiles
Compiled by: teacher
2012
The structure "STUDENT'S REFERENCE ON ORGANIC CHEMISTRY "
EXPLANATORY NOTE
SS in organic chemistry is compiled to assist students in creating a scientific picture of the world through chemical content, taking into account intersubject and intrasubject connections, the logic of the educational process.
In the SS on organic chemistry, the minimum in volume, but functionally complete content for the development of the state standard is presented chemical education.
The SS in Organic Chemistry has two main functions:
I. The information function allows participants in the educational process to get an idea of \u200b\u200bthe content, structure of the subject, the relationship of concepts through diagrams, tables and algorithms.
II. Organizational and planning function provides for the selection of training stages, structuring of educational material, and creates an idea of \u200b\u200bthe content of the intermediate and final certification.
SS involves the formation of a system of knowledge, skills and methods of activity, develops the ability of students to work with reference materials.
Name | Name |
||
Chronological table "Development of organic chemistry". | Chemical properties of alkenes (ethylene hydrocarbons). |
||
Basic provisions of the theory of the structure of organic compounds | Chemical properties of alkynes (acetylenic hydrocarbons). |
||
Isomers and homologues. | Chemical properties of arenes (aromatic hydrocarbons). |
||
TCOC value | |||
Classification of hydrocarbons. | Genetic relationship of organic substances. |
||
Homological series ALKANES (LIMIT HYDROCARBONS). | Interconnection "Structure - properties - application". |
||
Homological series RADICAL FORMED FROM ALKANES. | Relative molecular weights of organic substances |
||
Dictionary of organic chemistry terms. Name reactions. |
|||
Isomerism of classes of organic substances. | Algorithm for solving problems. Physical quantities for solving problems. |
||
Chemical properties of alkanes (saturated hydrocarbons). | Derivation of compound formulas. Examples of problem solving. |
CHRONOLOGICAL TABLE "DEVELOPMENT OF ORGANIC CHEMISTRY"
Period / year. Who! | The nature of the opening |
|
Ancient-shy | Ancient man | Boil food, tan leather, make medicines |
Paracelsus and others. | Manufacturing more complex drugs, studying the properties of substances org. origin, i.e. waste products |
|
XY-XYIII c. in. | Continuous process | Accumulation of knowledge about various substances. The supremacy of "VITALISTIC PERCEPTIONS" |
An explosion of scientific thought, the detonator of which was the people's need for dyes, clothing, and food. |
||
Jones Jakob Berzelius (Swedish chemist) | The term "organic chemistry" |
|
Friedrich Wöhler (German) | Oxalic acid synthesis |
|
Concept | Organic chemistry is a branch of chemical science that studies carbon compounds. |
|
Friedrich Wöhler (German) | Synthesis of urea |
|
Aniline synthesis |
||
Adolf Kulbe (German) | Synthesis of acetic acid from carbon |
|
E. Frankland | The concept of "connecting system" - valence |
|
Pierre Berthelot (fr.) | He synthesized ethyl alcohol by hydration of ethylene. Synthesis of fats. "Chemistry does not need vitality!" |
|
Synthesis of sugar substance |
||
Based on various theories (Frankland, Gerard, Kekulé, Cooper) created the TSOC |
||
Textbook "Introduction to the complete study of organic chemistry." Organic chemistry is a branch of chemistry that studies hydrocarbons and their derivatives . |
MAIN PROVISIONS
THEORY OF STRUCTURE OF ORGANIC COMPOUNDS
A. M. BUTLEROVA
1. A. in M. are connected in a certain sequence, according to their valence.
2. The properties of substances depend not only on the qualitative and quantitative composition, but also on the chemical structure. Isomers. Isomerism.
3. A. and A. groups mutually influence each other.
4. By the properties of a substance, you can determine the structure, and by the structure - properties.
Isomers and homologues.
Qualitative composition | Quantitative composition | Chemical structure | Chemical properties |
|
Isomers | same | same | various | various |
Homologues | same | different | similar | similar |
TCOC value
1. Explained M.'s structure of known substances and their properties.
2. It made it possible to foresee the existence of unknown substances and find ways of their synthesis.
3. Explain the variety of organic substances.
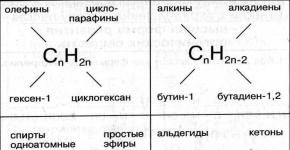
Classification of hydrocarbons.
https://pandia.ru/text/78/431/images/image003_147.gif "width \u003d" 708 "height \u003d" 984 src \u003d "\u003e
Homological series
ALKANES (MAXIMUM HYDROCARBONS)
Formula | Name |
METHANE |
|
C2H6 | ETHANE |
C3H8 | PROPANE |
BUTANE |
|
PENTANE |
|
HEXANUS |
|
HEPTANE |
|
OCTANE |
|
NONAN |
|
C10H22 | DEAN |
Homological series
RADICALS FORMED FROM ALKANES
Formula | Name |
METHYL |
|
C2H5 | ETHYL |
C3H7 | DROP |
BUTYL |
|
PENTIL |
|
HEXYL |
|
HEPTYL |
|
OCTIL |
|
NONIL |
|
C10H21 | DECIL |
General information about hydrocarbons.
DIV_ADBLOCK54 "\u003e

Chemical properties of alkanes
(saturated hydrocarbons).
https://pandia.ru/text/78/431/images/image007_73.gif "width \u003d" 610 "height \u003d" 835 src \u003d "\u003e
Chemical properties of alkynes
(acetylene hydrocarbons).
https://pandia.ru/text/78/431/images/image009_68.gif "width \u003d" 646 "height \u003d" 927 src \u003d "\u003e
Genetic relationship between hydrocarbons.
https://pandia.ru/text/78/431/images/image011_36.jpg "width \u003d" 696 "height \u003d" 919 src \u003d "\u003e
Interrelation "Structure - properties - application". | The ways receiving |
||
| Structure | ||
Composition | Finding in nature | ||
| Properties | Application |
|
MOLECULAR MASSES OF SOME ORGANIC SUBSTANCES.
Name | ||||||||
Alkanes | ||||||||
Halogen derivatives | ||||||||
Alcohols and Phenols | ||||||||
Ethers | ||||||||
Aldehydes | ||||||||
Carboxylic acids | ||||||||
Nitro compounds | ||||||||
Algorithm for solving problems
1. Study carefully the conditions of the problem: determine with what values \u200b\u200bthe calculations are to be carried out, designate them with letters, set their units of measurement, numerical values, determine which value is the desired one.
2. Record the task data in short terms.
3. If the problem deals with the interaction of substances, write down the equation of the reaction (reactions) and equalize it (their) coefficients.
4. Find out the quantitative relationship between the task data and the desired value. To do this, divide your actions into stages, starting with the question of the problem, clarifying the pattern with which you can determine the desired value at the last stage of calculations. If the initial data lacks any values, think about how they can be calculated, that is, determine the preliminary stages of calculation. There can be several of these stages.
5. Determine the sequence of all stages of solving the problem, write down the necessary calculation formulas.
6. Substitute the corresponding numerical values \u200b\u200b\\ u200b \\ u200bof the quantities, check their dimensions, make calculations.


Derivation of compound formulas.
This type of calculation is extremely important for chemical practice, since it allows one to determine the formula of a substance (simplest and molecular) based on experimental data.
Based on the data of qualitative and quantitative analyzes, the chemist first finds the ratio of atoms in a molecule (or other structural unit of a substance), that is, its simplest formula.
For example, analysis showed that the substance is a hydrocarbon
CxHy, in which the mass fractions of carbon and hydrogen are 0.8 and 0.2, respectively (80% and 20%). To determine the ratio of atoms of elements, it is enough to determine their amount of substance (number of moles): Whole numbers (1 and 3) are obtained by dividing 0.2 by 0.0666. Let's take the number 0.0666 as 1. The number 0.2 is 3 times more than the number 0.0666. So CH3 is the simplest the formula of this substance. The ratio of C and H atoms, equal to 1: 3, corresponds to an infinite number of formulas: C2H6, C3H9, C4H12, etc., but from this series only one formula is molecular for a given substance, i.e., reflecting the true number of atoms in its molecule. To calculate the molecular formula, in addition to the quantitative composition of a substance, it is necessary to know its molecular weight.
To determine this value, the value of the relative density of gas D is often used. Thus, for the above case, DH2 \u003d 15. Then M (CxHy) \u003d 15µM (H2) \u003d 152 g / mol \u003d 30 g / mol.
Since M (CH3) \u003d 15, the indices in the formula must be doubled to match the true molecular weight. Hence, molecular substance formula: C2H6.
Determination of the formula of a substance depends on the accuracy of mathematical calculations.
When finding the value n element should take into account at least two decimal places and carefully round the numbers.
For example, 0.8878 ≈ 0.89, but not 1. The ratio of atoms in a molecule is not always determined by simply dividing the numbers obtained by a smaller number.
by mass fractions of elements.
Task 1. Establish the formula of a substance that consists of carbon (w \u003d 25%) and aluminum (w \u003d 75%).

Divide 2.08 by 2. The resulting number 1.04 does not fit an integer number of times in the number 2.78 (2.78: 1.04 \u003d 2.67: 1).
Now let's divide 2.08 by 3.
In this case, the number 0.69 is obtained, which fits exactly 4 times in the number 2.78 and 3 times in the number 2.08.
Therefore, the indices x and y in the formula of the substance AlxCy are 4 and 3, respectively.
Answer: Al4C3 (aluminum carbide).
Algorithm for finding the chemical formula of a substance
by its density and mass fractions of elements.
A more complex version of the problem of deriving the formulas of compounds is the case when the composition of a substance is specified through the combustion products of these.
Problem 2. Combustion of a hydrocarbon weighing 8.316 g produced 26.4 g of CO2. The density of the substance under normal conditions is 1.875 g / ml. Find its molecular formula.

General information about hydrocarbons.
(continued)
https://pandia.ru/text/78/431/images/image025_32.gif "width \u003d" 696 "height \u003d" 983 "\u003e
Natural sources of hydrocarbons.
Oil - fossil, liquid fuel, a complex mixture of organic substances: saturated hydrocarbons, paraffins, naphthenes, aromatics, etc. Oil usually contains oxygen, sulfur and nitrogen-containing substances.
Oily liquid with a characteristic odor, dark in color, lighter than water. The most important source of fuel, lubricating oils and other petroleum products. The main (primary) refining process is distillation, as a result of which gasoline, naphtha, kerosene, diesel oils, fuel oil, petroleum jelly, paraffin, and tar are obtained. Secondary processing processes ( cracking, pyrolysis) allow you to get additional liquid fuel, aromatic hydrocarbons (benzene, toluene, etc.), etc.
Petroleum gases - a mixture of various gaseous hydrocarbons dissolved in oil; they are released during extraction and processing. They are used as fuel and chemical raw materials.
Petrol - colorless or yellowish liquid, consists of a mixture of hydrocarbons ( C5 - C11 ). It is used as a motor fuel, solvent, etc.
Naphtha - transparent yellowish liquid, mixture of liquid hydrocarbons. It is used as diesel fuel, solvent, hydraulic fluid, etc.
Kerosene - transparent, colorless or yellowish liquid with a blue tint. It is used as fuel for jet engines, for domestic needs, etc.
Solarium - yellowish liquid. It is used for the production of lubricating oils.
Fuel oil - heavy fuel oil, a mixture of paraffins. They are used in the production of oils, heating oil, bitumen, for processing into light motor fuel.
Benzene - colorless mobile liquid with a characteristic odor. Used for the synthesis of organic compounds, as a raw material for the production of plastics, as a solvent, for the production of explosives, in the aniline paint industry
Toluene - analogue of benzene. They are used in the production of caprolactam, explosives, benzoic acid, saccharin, as a solvent, in the aniline paint industry, etc.
Lubricating oils - Used in various fields of technology to reduce friction fur. parts, to protect metals from corrosion, as a lubricating fluid.
Tar - black resinous mass. It is used for lubrication, etc.
Petrolatum - a mixture of mineral oil and paraffins. They are used in electrical engineering, for lubricating bearings, for protecting metals from corrosion, etc.
Paraffin - a mixture of solid saturated hydrocarbons. Used as an electrical insulator, in chem. industry - for obtaining higher acids and alcohols, etc.
Plastic - materials based on high molecular weight compounds. They are used for the production of various technical products and household items.
Asphalt ore - a mixture of oxidized hydrocarbons. It is used for the manufacture of varnishes, in electrical engineering, for asphalting streets.
Mountain wax - a mineral from the group of petroleum bitumen. Used as an electrical insulator, for the preparation of various lubricants and ointments, etc.
Artificial wax- refined mountain wax.
Coal - solid fuel fossil of plant origin, black or black and gray. Contains 75–97% carbon. It is used as a fuel and as a raw material for the chemical industry.
Coke - a caked solid product formed when some coals are heated in coke ovens to 900-1050 °C. Used in blast furnaces.
Coke oven gas - gaseous products of coking of fossil coals. Consists of CH4, H2, CO and others, also contains non-combustible impurities. It is used as a high-calorific fuel.
Ammonia water - a liquid product of dry distillation of coal. It is used to obtain ammonium salts (nitrogen fertilizers), ammonia, etc.
Coal tar - a thick, dark liquid with a characteristic odor, a product of dry distillation of coal. It is used as a raw material for chemical. industry.
Benzene - a colorless mobile liquid with a characteristic odor, one of the products of coal tar. They are used for the synthesis of organic compounds, as explosives, as a raw material for the production of plastics, as a dye, as a solvent, etc.
Naphthalene - a solid crystalline substance with a characteristic odor, one of the products of coal tar. Derivatives of naphthalene are used to obtain dyes and explosives, etc.
Medicines - the by-product coke industry provides a number of drugs (carbolic acid, phenacitin, salicylic acid, saccharin, etc.).
Pitch - solid (viscous) mass of black color, the residue from the distillation of coal tar. Used as a waterproofing agent, for the production of fuel briquettes, etc.
Toluene - analogue of benzene, one of the products of coal tar. They are used for the production of explosives, caprolactam, benzoic acid, saccharin, as a dye, etc.
Dyes - one of the products of coke-chemical production, obtained as a result of the processing of benzene, naphthalene and phenol. Used in the national economy.
Aniline - colorless oily liquid, poisonous. It is used to obtain various organic substances, aniline dyes, various azo dyes, the synthesis of medicinal preparations, etc.
Saccharin - a solid white crystalline substance of sweet taste, obtained from toluene. It is used instead of sugar for diabetes, etc.
BB - derivatives of coal obtained in the process of dry distillation. They are used in the military industry, mining and other sectors of the national economy.
Phenol - a crystalline substance of white or pink color with a characteristic strong odor. It is used in the production of phenol-formaldehyde plastics, synthetic nylon fiber, dyes, drugs, etc.
Plastic - materials based on high molecular weight compounds. They are used for the production of various technical products and household items.
State budgetary educational institution of higher professional education
"Pyatigorsk State Pharmaceutical Academy"
Ministry of Health and Social Development of the Russian Federation
ORGANIC CHEMISTRY
DIAGRAMS AND DRAWINGS
Textbook for 2nd year students (3, 4 semesters)
(full-time education) for students of 2 and 3 courses (correspondence education)
on discipline С2.B.7 - "Organic chemistry"
Pyatigorsk, 2011
UDC. 547 (076)
Published by the decision of the CMS of the Pyatigorsk State Pharmaceutical Academy. Minutes No. 7 dated 02.04.2003
General edition: Head. department, professor Oganesyan E.T.
But on the basis of the current program in organic chemistry for pharmaceutical universities, a manual has been created that allows you to obtain information in a concise and accessible form about the structure, methods of production and reactivity of the most important classes of organic compounds.
Reviewers: Professor V.A. Kompantsev, Associate Professor A.S. Saushkina
Editorial Council:
Belikov V.G. (Responsible editor) - prof. Doctor of Philosophy; Vergeichik E.N. (deputy editor) - prof., Doctor of Philosophy; V.I. Pogorelov (deputy editor) - prof., Doctor of Philosophy; Muravyova D.A. - Prof., Doctor of Philosophy; Gayevy M.D. - prof., Doctor of medical sciences; V.V. Gatsan - prof., Ph.D.
V.V. Karpova; Bratashova T.M. (responsible secretary)
1.1 Classification and main varieties of the nomenclature
1.3 Substitutional nomenclature for functional derivatives
2.2 sp 3 -Hybridization. Alkane structure. Forecasting
2.3 The structure of cycloalkanes. Predicting reactionary
2.4 sp 2 -Hybridization. Ethylene structure. Forecasting
2.5 The structure of butadiene-1,3. Pairing concept. Influence
2.7 sp-hybridization. Acetylene structure and reaction
ability of alkynes ................................................ ............................................... |
|||
Electronic structure of heterocyclic compounds. |
|||
Prediction of reactivity based on structural analysis ............. |
|||
Features of the structure of the sp2 -hybrid nitrogen atom .................................... |
|||
Electronic structure of pyridine ............................................... .................... |
|||
Electronic structure of pyrrole ............................................... ...................... |
|||
Electronic structure of pyrazole ............................................... .................... |
|||
Isomerism of organic compounds ............................................... ........................ |
|||
Types of isomerism ................................................ ................................................ |
|||
Properties of chiral compounds ............................................... ................... |
|||
Rules for working with Fisher's projection formulas ............................. |
|||
Stereochemical nomenclature ................................................ ............................ |
|||
D-, L-notation system ........................................... .................................. |
|||
R-, S-notation system ........................................... .................................. |
|||
Classification and mechanisms of organic reactions ........................................... |
|||
Classification of reactions ................................................ ................................. |
|||
The mechanism of reactions of radical substitution (SR) ....................................... |
|||
Mechanism of electrophilic substitution (SE) reactions ................................ |
|||
The mechanism of the reaction of nucleophilic substitution (SN) in |
|||
sp3 -hybrid carbon atom ............................................. ................................. |
|||
Mechanism of reactions of electrophilic addition (AdE) ........................ |
|||
Mechanism of nucleophilic addition reactions (AdN) .......................... |
|||
Reactivity and methods of obtaining organic substances in |
|||
schemes ................................................. .................................................. .......................... |
|||
FOREWORD
The study of organic chemistry in pharmaceutical higher educational institutions sets as its most important goal the formation of a methodological approach for students to study the relationship between the structure of molecules and their properties.
The abundance of theoretical material creates the prerequisites for achieving this goal, however, students often feel an urgent need for such a source of information that would make it possible to easily and quickly answer many questions related to the study of methods of obtaining and the reactivity of organic compounds.
This study guide is precisely designed to help students get information in a concise and accessible form,
concerning the structure and properties of the most important classes of organic compounds.
1. BASES OF CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
1.1 Classification and main varieties of the nomenclature of organic compounds
Organic chemistryis the chemistry of hydrocarbons and their derivatives. Several million organic compounds are now known. To study such a huge number of substances, they are divided into smaller groups - classes within which the compounds have similarities in structure, and hence in chemical properties.
Organic substances can be classified according to different criteria: I - according to the structure of the carbon chain, they can be a) acyclic (carbon
parent chains do not have cycles); b) cyclic (carbon chains are closed in cycles);
II - by the nature of carbon-carbon bonds, substances are divided into a) limiting (in molecules only single carbon-carbon bonds); b) unsaturated (molecules have double or triple carbon-carbon bonds); c) aromatic (cyclic compounds with a special type of bond (see.
III - according to the presence of functional groups, substances are assigned to different classes (the most important are presented in Table 1).
A nomenclature is a set of rules for giving a name to each chemical compound. The replacement nomenclature is of the greatest importance; for hydrocarbon derivatives, in addition to the substituent one, the radical-functional nomenclature is often used. For some compounds, trivial (historical) names are used.
1.2 Substitutional nomenclature of hydrocarbons
Hydrocarbons are substances whose molecules are composed only of carbon and hydrogen atoms.
To give a name to an acyclic hydrocarbon according to the substituent nomenclature, it is necessary:
1 . Select the parent structure using the following order:
1) the maximum number of multiple (double, triple) bonds;
2) maximum chain length;
3) the maximum number of substituents (radicals).
2 *. Number the parent structure so that the smallest values \u200b\u200b(locants) are:
1) multiple connections;
2) hydrocarbon substituents.
Each subsequent item is valid in the absence of the previous one, or if the previous one did not give an unambiguous answer.
3. Name all radicals (see Table 2)
4. Compose the title according to the following scheme:
Prefix |
Ending |
||||||||||||||||||||||||||||||||||||||
Hydrocarbon |
An - alkanes |
||||||||||||||||||||||||||||||||||||||
alternates |
hydrocarbon |
En - alkenes |
indicating |
||||||||||||||||||||||||||||||||||||
alphabetically |
chain (progenitor |
Yn - alkynes |
provisions |
||||||||||||||||||||||||||||||||||||
structure) |
Diene - alkadienes |
multiple links |
|||||||||||||||||||||||||||||||||||||
For example: |
|||||||||||||||||||||||||||||||||||||||
3-ethylhexane |
|||||||||||||||||||||||||||||||||||||||
C2 H5 |
|||||||||||||||||||||||||||||||||||||||
3-methyl-3-ethylpentene-1 |
|||||||||||||||||||||||||||||||||||||||
CH3 2 |
|||||||||||||||||||||||||||||||||||||||
(CH2) |
|||||||||||||||||||||||||||||||||||||||
C3 H7 CH3 |
|||||||||||||||||||||||||||||||||||||||
3,3,4-trimethyl-4-propylnonine-1 |
|||||||||||||||||||||||||||||||||||||||
2-isopropyl-butadiene-1,3 or 2- (1-methylethyl) butadiene-1,3 |
|||||||||||||||||||||||||||||||||||||||
Table 1 |
|||||||||||||||||||||||||||||||||||||||


table 2 |
|||||||
Names of some hydrocarbon substituents |
|||||||
Names |
|||||||
trivial, |
systematic |
||||||
permissible |
|||||||
CH3 - |
|||||||
(C H -) |
|||||||
isopropyl |
1-methylethyl |
||||||
CH3 -CH2 -CH2 -CH2 - |
|||||||
CH CH2 |
isobutyl |
2-methylpropyl |
|||||
sec-butyl |
1-methylpropyl |
||||||
tert-butyl |
1,1-dimethylethyl |
||||||
II Alkenyls |
|||||||
CH2 - |
propen-2-yl |
||||||
III Alkynyls |
|||||||
not used |
|||||||
C CH2 - |
not used |
propyn-2-yl |
|||||
(C6 H5 -) |
|||||||
2-methylphenyl |
|||||||
phenylmethyl |
|||||||
2-phenylethenyl |
|||||||

For cyclic hydrocarbons, either a cycle or an acyclic hydrocarbon chain linked to the cycle is selected as the parent structure. The numbering of the cycle in the case of the presence of substituents is made from one substituent to another so that the locants get the lowest value.
CH2 -CH2 -CH3 |
CH C2 H5 |
|||||||||||||
sec-butylbenzene |
||||||||||||||
1-methyl-2-propylcyclopentane |
||||||||||||||
For some cyclic hydrocarbons, the IUPAC rules allow the following trivial names:
C CH3 |
|||||||||
ortho-xylene |
meta-xylene |
||||||||
para-xylene |
|||||||||
naphthalene |
|||||||||
anthracene |
|||||||||
phenanthrene |
|||||||||
H3 C C CH3 |
|||||||||

1.3 Substitute nomenclature of functional hydrocarbon derivatives
Functional groups (F.G.) - groups of atoms of non-carbon |
||||||
character replacing hydrogen atoms in the hydrocarbon chain and |
||||||
defining properties (function) of compounds. |
||||||
The most important functional groups are: |
||||||
Table 3 |
||||||
Name |
Name |
Name |
||||
hydroxy- |
||||||
SO3 H |
||||||
carbonyl- |
||||||
alkylthio |
||||||
carboxyl- |
||||||
carbamoyl- |
||||||
carbonyl- |
||||||
By the nature and amount of FG, organic compounds are divided into the following |
||||||
other groups: |
||||||
Functional derivatives of hydrocarbons
Monofunctional |
Multifunctional |
Heterofunctional |
|||||||||||||||||||||
identical F.G.) |
|||||||||||||||||||||||
To give a name to functional derivatives of hydrocarbons, one must: 1. Select the parent structure - a hydrocarbon chain linked by:
1) with a functional group (for monofunctional compounds);
2) with a large number of functional groups (for polyfunctional compounds);
With new programs and textbooks, this issue becomes most acute. Our school switched to new textbooks by O.S. Gabrielyan and the new program, like most schools in the Zavolzhsky region, therefore we present calendar-thematic planning for the course "Organic Chemistry" Grade 10. Thematic planning is compiled according to the program developed by the Department of Educational Programs and ...
Activity. The search for methods and forms of teaching that contribute to the education of a creative personality has led to the emergence of some specific teaching methods, one of which is play methods. The implementation of game teaching methods in the study of chemistry in the conditions of adherence to didactic and psychological-pedagogical characteristics, increases the level of training of students. The word "game" in Russian ...




Secondly, at present, a sufficient number of compounds are known that are insoluble in non-polar solvents or, conversely, readily soluble in water, which, nevertheless, are classified as lipids. In modern organic chemistry, the definition of the term "lipids" is based on the biosynthetic relationship of these compounds - fatty acids and their derivatives are referred to as lipids. At the same time in biochemistry ...
The work is intended for teachers of chemistry, and can also be useful for students of pedagogical universities and colleges. 2.2. EXPLANATORY NOTE The need to develop an elective course for 10th grade students "Solving problems in organic chemistry of an increased level of complexity" is due to several reasons. In accordance with the basic curriculum of a complete high school for the study of chemistry in 2 ...
State budgetary educational institution of higher professional education
"Pyatigorsk State Pharmaceutical Academy"
Ministry of Health and Social Development of the Russian Federation
ORGANIC CHEMISTRY
DIAGRAMS AND DRAWINGS
Textbook for 2nd year students (3, 4 semesters)
(full-time education) for students of 2 and 3 courses (correspondence education)
on discipline С2.B.7 - "Organic chemistry"
Pyatigorsk, 2011
UDC. 547 (076)
Published by the decision of the CMS of the Pyatigorsk State Pharmaceutical Academy. Minutes No. 7 dated 02.04.2003
General edition: Head. department, professor Oganesyan E.T.
But on the basis of the current program in organic chemistry for pharmaceutical universities, a manual has been created that allows you to obtain information in a concise and accessible form about the structure, methods of production and reactivity of the most important classes of organic compounds.
Reviewers: Professor V.A. Kompantsev, Associate Professor A.S. Saushkina
Editorial Council:
Belikov V.G. (Responsible editor) - prof. Doctor of Philosophy; Vergeichik E.N. (deputy editor) - prof., Doctor of Philosophy; V.I. Pogorelov (deputy editor) - prof., Doctor of Philosophy; Muravyova D.A. - Prof., Doctor of Philosophy; Gayevy M.D. - prof., Doctor of medical sciences; V.V. Gatsan - prof., Ph.D.
V.V. Karpova; Bratashova T.M. (responsible secretary)
1.1 Classification and main varieties of the nomenclature
1.3 Substitutional nomenclature for functional derivatives
2.2 sp 3 -Hybridization. Alkane structure. Forecasting
2.3 The structure of cycloalkanes. Predicting reactionary
2.4 sp 2 -Hybridization. Ethylene structure. Forecasting
2.5 The structure of butadiene-1,3. Pairing concept. Influence
2.7 sp-hybridization. Acetylene structure and reaction
ability of alkynes ................................................ ............................................... |
|||
Electronic structure of heterocyclic compounds. |
|||
Prediction of reactivity based on structural analysis ............. |
|||
Features of the structure of the sp2 -hybrid nitrogen atom .................................... |
|||
Electronic structure of pyridine ............................................... .................... |
|||
Electronic structure of pyrrole ............................................... ...................... |
|||
Electronic structure of pyrazole ............................................... .................... |
|||
Isomerism of organic compounds ............................................... ........................ |
|||
Types of isomerism ................................................ ................................................ |
|||
Properties of chiral compounds ............................................... ................... |
|||
Rules for working with Fisher's projection formulas ............................. |
|||
Stereochemical nomenclature ................................................ ............................ |
|||
D-, L-notation system ........................................... .................................. |
|||
R-, S-notation system ........................................... .................................. |
|||
Classification and mechanisms of organic reactions ........................................... |
|||
Classification of reactions ................................................ ................................. |
|||
The mechanism of reactions of radical substitution (SR) ....................................... |
|||
Mechanism of electrophilic substitution (SE) reactions ................................ |
|||
The mechanism of the reaction of nucleophilic substitution (SN) in |
|||
sp3 -hybrid carbon atom ............................................. ................................. |
|||
Mechanism of reactions of electrophilic addition (AdE) ........................ |
|||
Mechanism of nucleophilic addition reactions (AdN) .......................... |
|||
Reactivity and methods of obtaining organic substances in |
|||
schemes ................................................. .................................................. .......................... |
|||
FOREWORD
The study of organic chemistry in pharmaceutical higher educational institutions sets as its most important goal the formation of a methodological approach for students to study the relationship between the structure of molecules and their properties.
The abundance of theoretical material creates the prerequisites for achieving this goal, however, students often feel an urgent need for such a source of information that would make it possible to easily and quickly answer many questions related to the study of methods of obtaining and the reactivity of organic compounds.
This study guide is precisely designed to help students get information in a concise and accessible form,
concerning the structure and properties of the most important classes of organic compounds.
1. BASES OF CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDS
1.1 Classification and main varieties of the nomenclature of organic compounds
Organic chemistryis the chemistry of hydrocarbons and their derivatives. Several million organic compounds are now known. To study such a huge number of substances, they are divided into smaller groups - classes within which the compounds have similarities in structure, and hence in chemical properties.
Organic substances can be classified according to different criteria: I - according to the structure of the carbon chain, they can be a) acyclic (carbon
parent chains do not have cycles); b) cyclic (carbon chains are closed in cycles);
II - by the nature of carbon-carbon bonds, substances are divided into a) limiting (in molecules only single carbon-carbon bonds); b) unsaturated (molecules have double or triple carbon-carbon bonds); c) aromatic (cyclic compounds with a special type of bond (see.
III - according to the presence of functional groups, substances are assigned to different classes (the most important are presented in Table 1).
A nomenclature is a set of rules for giving a name to each chemical compound. The replacement nomenclature is of the greatest importance; for hydrocarbon derivatives, in addition to the substituent one, the radical-functional nomenclature is often used. For some compounds, trivial (historical) names are used.
1.2 Substitutional nomenclature of hydrocarbons
Hydrocarbons are substances whose molecules are composed only of carbon and hydrogen atoms.
To give a name to an acyclic hydrocarbon according to the substituent nomenclature, it is necessary:
1 . Select the parent structure using the following order:
1) the maximum number of multiple (double, triple) bonds;
2) maximum chain length;
3) the maximum number of substituents (radicals).
2 *. Number the parent structure so that the smallest values \u200b\u200b(locants) are:
1) multiple connections;
2) hydrocarbon substituents.
Each subsequent item is valid in the absence of the previous one, or if the previous one did not give an unambiguous answer.
3. Name all radicals (see Table 2)
4. Compose the title according to the following scheme:
Prefix |
Ending |
||||||||||||||||||||||||||||||||||||||
Hydrocarbon |
An - alkanes |
||||||||||||||||||||||||||||||||||||||
alternates |
hydrocarbon |
En - alkenes |
indicating |
||||||||||||||||||||||||||||||||||||
alphabetically |
chain (progenitor |
Yn - alkynes |
provisions |
||||||||||||||||||||||||||||||||||||
structure) |
Diene - alkadienes |
multiple links |
|||||||||||||||||||||||||||||||||||||
For example: |
|||||||||||||||||||||||||||||||||||||||
3-ethylhexane |
|||||||||||||||||||||||||||||||||||||||
C2 H5 |
|||||||||||||||||||||||||||||||||||||||
3-methyl-3-ethylpentene-1 |
|||||||||||||||||||||||||||||||||||||||
CH3 2 |
|||||||||||||||||||||||||||||||||||||||
(CH2) |
|||||||||||||||||||||||||||||||||||||||
C3 H7 CH3 |
|||||||||||||||||||||||||||||||||||||||
3,3,4-trimethyl-4-propylnonine-1 |
|||||||||||||||||||||||||||||||||||||||
2-isopropyl-butadiene-1,3 or 2- (1-methylethyl) butadiene-1,3 |
|||||||||||||||||||||||||||||||||||||||
Table 1 |
|||||||||||||||||||||||||||||||||||||||


table 2 |
|||||||
Names of some hydrocarbon substituents |
|||||||
Names |
|||||||
trivial, |
systematic |
||||||
permissible |
|||||||
CH3 - |
|||||||
(C H -) |
|||||||
isopropyl |
1-methylethyl |
||||||
CH3 -CH2 -CH2 -CH2 - |
|||||||
CH CH2 |
isobutyl |
2-methylpropyl |
|||||
sec-butyl |
1-methylpropyl |
||||||
tert-butyl |
1,1-dimethylethyl |
||||||
II Alkenyls |
|||||||
CH2 - |
propen-2-yl |
||||||
III Alkynyls |
|||||||
not used |
|||||||
C CH2 - |
not used |
propyn-2-yl |
|||||
(C6 H5 -) |
|||||||
2-methylphenyl |
|||||||
phenylmethyl |
|||||||
2-phenylethenyl |
|||||||

For cyclic hydrocarbons, either a cycle or an acyclic hydrocarbon chain linked to the cycle is selected as the parent structure. The numbering of the cycle in the case of the presence of substituents is made from one substituent to another so that the locants get the lowest value.
CH2 -CH2 -CH3 |
CH C2 H5 |
|||||||||||||
sec-butylbenzene |
||||||||||||||
1-methyl-2-propylcyclopentane |
||||||||||||||
For some cyclic hydrocarbons, the IUPAC rules allow the following trivial names:
C CH3 |
|||||||||
ortho-xylene |
meta-xylene |
||||||||
para-xylene |
|||||||||
naphthalene |
|||||||||
anthracene |
|||||||||
phenanthrene |
|||||||||
H3 C C CH3 |
|||||||||

1.3 Substitute nomenclature of functional hydrocarbon derivatives
Functional groups (F.G.) - groups of atoms of non-carbon |
||||||
character replacing hydrogen atoms in the hydrocarbon chain and |
||||||
defining properties (function) of compounds. |
||||||
The most important functional groups are: |
||||||
Table 3 |
||||||
Name |
Name |
Name |
||||
hydroxy- |
||||||
SO3 H |
||||||
carbonyl- |
||||||
alkylthio |
||||||
carboxyl- |
||||||
carbamoyl- |
||||||
carbonyl- |
||||||
By the nature and amount of FG, organic compounds are divided into the following |
||||||
other groups: |
||||||
Functional derivatives of hydrocarbons
Monofunctional |
Multifunctional |
Heterofunctional |
|||||||||||||||||||||
identical F.G.) |
|||||||||||||||||||||||
To give a name to functional derivatives of hydrocarbons, one must: 1. Select the parent structure - a hydrocarbon chain linked by:
1) with a functional group (for monofunctional compounds);
2) with a large number of functional groups (for polyfunctional compounds);
This manual contains in a visual form the course of organic chemistry, studied in grades 10-11 of a comprehensive school. The manual can be used in the study, generalization and repetition of educational material, and can also be useful in organizing systematic repetition in preparation for final or entrance exams.
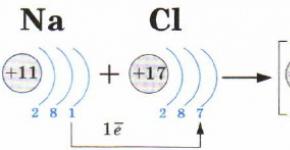
The theory of radicals (30 years of the XIX century J. Berzelius, J. Liebig, J. Dumas)
a) organic substances include radicals;
b) radicals are always constant, do not undergo changes, pass from one molecule to another;
c) radicals can exist in free form.
The concept of "radical" has become firmly established in chemistry. The theory was subsequently rejected.
The theory of types (40-50 years of the XIX century.Ch. Gerard, A. Kekule and others)
a) all organic substances are derivatives of the simplest inorganic - such as hydrogen, water, ammonia, etc.
b) the formulas express not the internal structure of the molecule, but the methods of formation, the properties determine all the atoms of the molecule.
c) it is impossible to know the structure of matter, each substance has as many formulas as there are transformations.
The theory made it possible to classify organic substances, predict and discover some, special attention - to chemical transformations, but could not predict, indicate the ways of synthesis of new substances.
Content
I. Theory of the chemical structure of organic compounds
1 The emergence of organic chemistry as a science (1807 G. Berzelius) 3
2. Substances, organic and inorganic. Composition and some properties of organic substances 4
3. constructive theories 5
4. Relationship between the concepts of the theory of chemical structure 6
5. Preconditions for the emergence of the theory of chemical structure of organic substances 7
6. The theory of chemical structure. Fundamentals (1,2) 8
7. The theory of chemical structure. Fundamentals (3.4) 9
8. Theory of chemical structure. Fundamentals (5) 10
9. Algorithm for the search for possible alkane isomers (carbon skeleton isomerism) 11
10. Classification of chemical compounds typical for organic compounds (by the type of chemical transformation) 12
11. Classification of chemical compounds typical for organic compounds (by the type of bond breaking) 13
12. Classification of hydrocarbons 14
II. Saturated hydrocarbons
1. Methane. Physical properties. Molecule structure 15
2. Br3-hybridization 16
3. Alkanes 17
4. Isomers and homologues 18
5. Alkanes (unbranched structure) and alkyls 19
6. Nomenclature (rational) 20
7. Nomenclature (systematic) 21
8. Determination of the qualitative composition of organic compounds 22
9. Chemical properties of alkanes 23
10. Obtaining alkanes 24
11. Application of alkanes 25
12. Cycloalkanes (cycloparaffins, naphthenes) 26
III. Unsaturated hydrocarbons
1. Ethylene (ethene). Molecule structure. sp2 -hybridization 27
2. Alkenes (olefins, ethylene hydrocarbons) 28
3. Properties of alkenes 29
4. Properties of alkenes 30
5. Application of alkenes 31
6. Obtaining alkenes 32
7. Diene hydrocarbons (alkadienes) 33
8. Chemical properties of alkadienes (with conjugated bonds) Preparation 34
9. General characteristics of rubbers. Their structure and properties 35
10. Acetylene (ethine). Molecule structure sp-hybridization 36
11. Comparison of the structure of the ethane, ethylene and acetylene salt. Comparison of o and tc bonds 37
12. Alkines (acetylenic hydrocarbons) 38
13. Chemical properties of alkynes 39
14. Chemical properties of alkynes 40
15. Use of acetylene 41
16. Obtaining acetylene and its homologues 42
IV. Aromatic hydrocarbons
1. Benzene. Physical properties. Formula Kekule 43
2. Electronic structure of benzene 44
3. Chemical properties of benzene 45
4. Chemical properties of benzene 46
5. Arenas (Aromatic hydrocarbons. Alkylbenzenes) 47
6. Toluene. Chemical properties. Mutual influence of atoms in a toluene molecule 48
7. Rules of orientation in the benzene ring 49
8. The use of benzene. Acquiring 50 arenas
9. Styrene. Naphthalene. Anthracene 51
10. Genetic relationship between groups of hydrocarbons 52
11. General information about hydrocarbon groups 53
12. General information about groups of hydrocarbons 54
V. Alcohols and phenols
1. Saturated monohydric alcohols 55
2. Chemical properties of alcohols 56
3. Ethanol (Ethyl alcohol) 57
4. Application of saturated monohydric alcohols 58
5. Methods for obtaining alcohols 59
6. Saturated polyhydric alcohols 60
7. Ethers 61
8. Phenols 62
9. Chemical properties of phenol (hydroxyl group) 63
10. Chemical properties of phenol (on the benzene ring) 64
Vi. Aldehydes and carboxylic acids
1. Aldehydes. Structure. Nomenclature. Isomerism 65
2. Formaldehyde. Receiving. Properties 66
3. Properties of aldehydes 67
4. Properties of aldehydes 60
5. Ketones G9
6. Obtaining aldehydes and ketones 70
7. Carboxylic acids. Homological series 71
8. Some saturated monobasic acids 72
9. Carboxylic acids. Properties 73
10. Chemical properties of saturated monobasic carboxylic acids 74
11.Chemical properties of saturated monobasic carboxylic acids 15
12.Preparation of carboxylic acids 76
13.0 are separate representatives of carboxylic acids. Classification 77
14. Certain representatives of carboxylic acids 78
Vii. Esters. Fats
1. Esters 79
2. Chemical properties of esters 80
3. Fats. Classification. Obtaining 81
4. Chemical properties of fats 82
5. Soaps 83
6. Synthetic detergents (CMC) 84
VIII. Hydrocarbons
1. Carbohydrates. Composition. Classification 85
2. Glucose. Structure. Fructose 86
3. Glucose. Chemical properties 87
4. Glucose. Special properties. Application 88
5. Sucrose. Structure. Properties 89
6. Polysaccharides (CeH-mOsJn. Natural polymers 90
7. Starch and cellulose. Chemical properties 91
IX. Amines. Amino acids. Protein
1. Amines. Composition. Nomenclature. Isomerism 92
2. Amines. Chemical properties 93
3. Aniline. Structure. Properties 94
4. Amino acids. Nomenclature. Isomerism 95
5. Amino acids. Properties 96
6. Some amino acids of proteins 97
7. Obtaining and using amino acids 98
8. Proteins. Composition. Building 99
9. Protein structures 100
10. Chemical properties of proteins 101
11. Isomerism of classes of compounds 102
12. Genetic relationship of organic substances 103
X. Appendix
1. Qualitative reactions of organic compounds 104
2. Qualitative reactions of organic compounds 105
3. Periodic table of chemical elements 106
4. Symbols 107.
Free download the e-book in a convenient format, watch and read:
Download the book Chemistry in tables and diagrams, grades 10-11, Kovalevskaya N.B., 2007 - fileskachat.com, fast and free download.