The hardness of the water depends on what. Hardness of water
O.S.GABRIELYAN, T.N.PPKOVA,
G.A.SIVKOVA, S.A.SLADKOV
Water in our life
Teaching guide to the elective course
for grade 9 of basic school or 10-11 grades
basic high school
Continued. See the beginning in № 3, 4, 5, 6, 7, 8, 9, 10, 12, 13, 14, 15/2009
§ 12. HARDNESS OF WATER AND METHODS OF ELIMINATION
Purpose. Expand and deepen students' perceptions of exchange reactions, using information on how to eliminate water hardness. To cultivate respect for water resources.
The form of employment. The conversation, the story. Demonstration experiment.
Equipment and reagents. The device for receiving gases, a glass tube, test tubes, a tube holder; lime water, solutions of calcium chloride, hydrochloric acid, sodium carbonate, marble pieces.
Lesson plan
The concept of water hardness.
Types of water hardness.
Ways to eliminate water hardness.
Concept of water hardness
Absolutely pure water in nature does not exist. It always contains various impurities both in solution and in suspension, the suitability of water for domestic and industrial needs depends on their concentration and nature. For drinking water, strict standards for the content of elements, color, taste, etc. have been established. Thus, pH, an indicator of acidity or alkalinity of the environment, can be in the range of 6.5 to 9.5.
Certain requirements apply to water used in industry. For example, it should not spoil the product, cause corrosion of metal parts and mechanisms, clog pumps and pipes. Currently, more and more enterprises are striving to create closed cycles (cleaning - production - cleaning - production, etc.).
Water in which calcium and magnesium salts are dissolved has a special property - stiffness. It is known that it forms dense layers of scale (mainly calcium carbonate and sulphate and magnesium carbonate) on the walls of steam boilers, immersions, kettles. The salt build-up has a poor thermal conductivity, therefore it causes local overheating of the walls of the boiler and corrosion of the body. Accidental separation of part of the scale from the hot wall can cause rapid evaporation of water and even an explosion of the boiler.
Some food products are poorly soluble in hard water, vegetables boil down worse, the quality of cooked food decreases. When washing laundry in such water increases the consumption of detergents. Calcium-magnesium salts of higher carboxylic acids that make up the soap are deposited on the fabric. Threads are impregnated with them, products lose their former softness. Therefore, washing with soap and hard water requires preliminary softening of the water - elimination of hardness. Synthetic detergents provide a more efficient and economical washing process even in sea water, because they do not form insoluble calcium and magnesium salts.
Technologists at pharmaceutical and food factories monitor water quality especially carefully. When conducting quantitative analyzes, it is customary to express the hardness of water in millimole equivalents of calcium and magnesium ions contained in 1 liter of water:
1 mmol eq / l Ca 2+ corresponds to 20.04 mg / l;
1 mmol eq / l Mg 2+ corresponds to 12.16 mg / l.
In analytical practice, other methods of expressing concentration are used, for example, molar (mol / l). According to this indicator of water hardness it is divided into three groups:
- soft water having a hardness of up to 2 mmol eq / l;
- medium hard - from 2 to 10 mmol eq / l;
- hard - more than 10 mmol eq / l;
(mmol - millimole, one thousandth mole).
Types of water hardness
Different salts of calcium and magnesium cause different water hardness (see diagram).
Scheme
Ways to eliminate stiffness
In many areas of human activity need water with a certain content of dissolved salts in it. At water treatment plants using various methods of water purification and softening.
Elimination of hardness, or softening, of water consists in the removal of calcium and magnesium ions, which is carried out by three methods: thermal, chemical, and physico-chemical.
1. Thermal method - boiling water.
When boiling water, the decomposition of calcium and magnesium hydrogen carbonate occurs with the formation of carbon dioxide and carbonates of these metals, which precipitate:
Ca (HCO 3) 2 СaCO 3 + СО 2 + H 2 O,
Mg (HCO 3) 2 MgCO 3 H 2 O + CO 2.
Thus, as a result of boiling water, the rigidity due to the presence of hydrocarbons is eliminated. This is the so-called disposable (temporary) stiffness.
Thanks to this method, we can easily make fragrant tea or delicious compote. Vitamins and other beneficial compounds with soft water are better extracted from natural or dried fruit. Many housewives are aware of this and use pre-boiled water to prepare compotes, medicinal infusions, it is carefully drained without stirring up the carbonate residue.
2. Chemical methods.
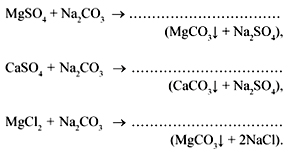
For some chemical purposes, a sufficiently complete purification of water from salts, which create rigidity, is required. For its elimination use chemical reagents, such as sodium carbonate, calcium hydroxide, sodium orthophosphate.
but) Lime soda method based on water treatment with hydrated lime, this eliminates temporary rigidity and also binds Fe 2+ ions.
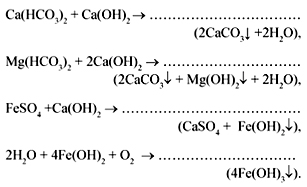
When adding soda, the permanent hardness is eliminated:
Students are encouraged to add their own reaction schemes.
The use of soda during washing saves detergents, and the absence of flocculent sediments improves the quality of hand and machine washing, especially the stains of various types of technical pollution are easily removed.
b) Phosphate method based on the formation of insoluble calcium and magnesium orthophosphates, precipitated:
3CaSO 4 + 2Na 3 PO 4 = 3Na 2 SO 4 + Ca 3 (PO 4) 2,
3MgCl 2 + 2Na 3 PO 4 = 6NaCl + Mg 3 (PO 4) 2.
Reagents, which form the basis of the chemical method of water softening, are part of the means that prevent the formation of scale on washing machines. Antiscale significantly prolong the life of expensive equipment, save energy by increasing the heat transfer of heating elements and improve the quality of washing, enhancing the effect of detergent.
Students can be offered the role of experts who evaluate the composition of reagents - anti-scaling indicated on the packaging. By careful reading, you can make sure that soda, phosphates and sodium polyphosphates are included as the main reagents that prevent the formation of scale.
3. Physico-chemical method.
It is based on the use of ion exchange resins - ion exchangers. They are solid polyelectrolytes in which ions of the same sign of charge are fixed on a solid matrix, and counter-ions are able to pass into solution.
Ionites used in ion-exchange installations are rather compact and are used in most modern filters for cleaning and softening water; they are equipped, for example, with food processing units of sea liners and submarines.
The ability to ion exchange manifests itself in a number of natural aluminosilicates. For example, when water is stirred with a small amount of clay, ions are exchanged between them. This effect is used for therapeutic purposes, clay applications are effective in the prevention of skin diseases, it restores the water-electrolyte balance.
Synthetic ion exchangers are widely used, they include polymeric materials. Depending on what ions pass into the solution from the surface, cation and anion exchangers are distinguished.
Cationites contain Na + or H + ions - these are sulphogli or aluminosilicates. Anionites include mobile hydroxide ions, these are so-called artificial resins.
Water softening in industrial ion exchangers is performed by filtration through a layer of cation exchanger 2–4 m thick, its surface is rather large, because The polymer base consists of small granules with a diameter of 0.5-1.5 mm. Calcium ions remain in the pores of these particles, instead of them sodium ions enter the water, and the water becomes soft.
The cation exchanger is periodically regenerated by washing it with a concentrated solution of sodium chloride, while calcium is forced to leave the previously occupied "positions" in the ion exchanger, its place is occupied by sodium ions. So update the adsorbent in the water filter "Geyser". For H + -cationites, washing is carried out with a solution of hydrochloric acid, and anion exchangers are regenerated with sodium hydroxide solution.
The demonstration experiment consists in obtaining hard water (the teacher passes carbon dioxide through a solution of lime water to dissolve the resulting sludge) and eliminate its hardness when heated or adding soda solution. Similarly, the removal of the constant hardness of water containing calcium chloride solutions is demonstrated.
Questions for conversation and fastening material
1. What ions determine the hardness of water and in what units is it measured?
2. Name the types of water hardness.
3. Why is it necessary to eliminate water hardness?
4. How does salt, which gives water hardness, affect the human body?
5. How to reduce the hardness of natural water in the home?
6. What are some ways to eliminate water hardness? Confirm your answer with chemical reaction equations.
7. Why soap is badly washed in hard water?
To be continued
"Hard" water- One of the most common problems, both in country houses with autonomous water supply, and in city apartments with centralized water supply. The degree of hardness depends on the presence of calcium and magnesium salts in water (hardness salt) and is measured in milligrams - equivalent per liter (mEq / l). According to the American classification (for drinking water) with a hardness of less than 2 mEq / l, the water is considered “soft”, from 2 to 4 mEq / l - normal (again, for food purposes!), From 4 to 6 mg -eq / l - hard, and over 6 mg-eq / l - very hard.
For many applications, water hardness does not play a significant role (for example, for extinguishing fires, watering a vegetable garden, cleaning streets and sidewalks). But in some cases, stiffness can create problems. When taking a bath, washing dishes, washing, washing machines hard water is much less effective than soft water. And that's why:
- When using soft water consumes 2 times less detergent;
- Hard water, interacting with soap, forms "soap slags" that do not wash off with water and leave little-like stains on the dishes and plumbing surfaces;
"Soap toxins" also do not wash off the surface of human skin, clogging the pores and covering every hair on the body, which can cause a rash, irritation, itching; - When water is heated, the hardness salts it contains crystallize, falling as scum. Scale causes 90% of water heater failures. Therefore, to the water subjected to heating in boilers, boilers, etc., more stringent requirements for stringency are imposed;
- In many industrial processes, hardness salts can enter into a chemical reaction, forming undesirable intermediates.
Stiffness concept
Water hardness is usually associated with calcium cations (Ca2 +) and to a lesser extent magnesium (Mg2 +). AT in fact, all divalent cations in varying degrees affect the rigidity. They interact with anions, forming compounds (salts of hardness) capable of precipitating. Monovalent cations (for example, sodium Na +) do not possess this property.
This table lists the main cations of metals that cause stiffness and the main anions with which they are associated.
In practice, strontium, iron and manganese have so little effect on hardness that they are usually neglected. Aluminum (Al3 +) and trivalent iron (Fe3 +) also affect stiffness, but at pH levels found in natural waters, their solubility and, accordingly, the “contribution” to stiffness is negligible. Similarly, the insignificant effect of barium (Ba2 +) is not taken into account.
Types of stiffness
Overall stiffness. Determined by the total concentration of calcium and magnesium ions. It is the sum of carbonate (temporary) and non-carbonate (constant) hardness.
Carbonate stiffness. Due to the presence of bicarbonates and carbonates (at pH\u003e 8.3) in water, calcium and magnesium. This type of hardness is almost completely eliminated by boiling water and therefore is called temporary hardness. When water is heated, the bicarbonates decompose to form carbonic acid and precipitate calcium carbonate and magnesium hydroxide.
Non-carbonate stiffness. Caused by the presence of calcium and magnesium salts of strong acids (sulfuric, nitric, hydrochloric) and when boiling is not eliminated (constant hardness).
Units
In world practice, several units of measurement of stiffness are used, all of them in a certain way correlate with each other. In Russia, Gosstandart sets mol per cubic meter (mol / m3) as a unit of water hardness.
One mole per cubic meter corresponds to a mass concentration of calcium ion equivalents (1/2 Ca2 +) 20.04 g / m3 and magnesium ions (1 / 2Mg2 +) 12.153 g / m3. The numerical value of stiffness, expressed in moles per cubic meter, is equal to the numerical value of stiffness, expressed in milligram equivalents per liter (or cubic decimeter), i.e. 1 mol / m3 = 1 mmol / l = 1 mg-equiv / l = 1 mg-equiv / dm3.
In addition, such stiffness units as German degree (do, dH), French degree (fo), American degree, ppm CaCO3 are widely used in foreign countries.
The ratio of these stiffness units is presented in the following table:
Note:
One German degree corresponds to 10 mg / dm3 CaO or 17.86 mg / dm3 CaCO3 in water.
One French degree corresponds to 10 mg / dm3 of CaCO3 in water.
One American degree corresponds to 1 mg / dm3 of CaCO3 in water.
Origin of stiffness
Calcium ions (Ca2 +) and magnesium (Mg2 +), as well as other alkaline earth metals, which cause hardness, are present in all mineralized waters. Their source is natural deposits of limestone, gypsum and dolomite. Calcium and magnesium ions enter the water as a result of the interaction of dissolved carbon dioxide with minerals and other processes of dissolution and chemical weathering of rocks. The source of these ions can also serve as microbiological processes occurring in the soils in the catchment area, in the bottom sediments, as well as the wastewater of various enterprises.
Water hardness varies widely and there are many types of water classification according to the degree of its hardness.
Usually in low-mineralized waters prevails (up to 70% -80%) rigidity due to calcium ions (although in some rare cases, magnesium hardness can reach 50-60%). With an increase in the degree of mineralization of water, the content of calcium ions (Ca2 +) decreases rapidly and rarely exceeds 1 g / l. The content of magnesium ions (Mg2 +) in highly mineralized waters can reach several grams, and in salt lakes - tens of grams per liter of water.
In general, the hardness of surface water is usually less than the hardness of groundwater. The hardness of surface waters is subject to noticeable seasonal fluctuations, reaching usually the highest value at the end of winter and the lowest during the flood period, when richly diluted with soft rain and melt water. Sea and ocean water have very high hardness (tens and hundreds of mEq / dm3)
Stiffness effect
From the point of view of drinking water use, its acceptability in terms of severity may vary significantly depending on local conditions. The taste threshold for calcium ion is (in terms of mg equivalent) in the range of 2-6 mg-eq / l, depending on the corresponding anion, and the taste threshold for magnesium is lower. In some cases, water with hardness above 10 mEq / L is acceptable for consumers. High hardness affects the organoleptic properties of water, giving it a bitter taste and having a negative effect on the digestive organs
The World Health Organization does not offer any recommended amount of hardness for indications of health effects. The WHO materials state that although a number of studies have revealed a statistically inverse relationship between drinking water hardness and cardiovascular diseases, the available data are not sufficient to conclude that this relationship is causal. Similarly, it is not unequivocally proven that soft water has a negative effect on the balance of minerals in the human body.
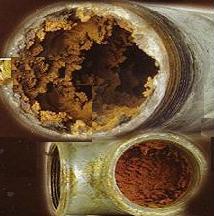 However, depending on pH and alkalinity, water with a hardness above 4 mEq / l can cause a deposit of slag and scale (calcium carbonate) in the distribution system, especially when heated. That is why the requirements of Kotlonadzor introduce very stringent requirements for the amount of hardness of water used to power boilers (0.05–0.1 mEq / l).
However, depending on pH and alkalinity, water with a hardness above 4 mEq / l can cause a deposit of slag and scale (calcium carbonate) in the distribution system, especially when heated. That is why the requirements of Kotlonadzor introduce very stringent requirements for the amount of hardness of water used to power boilers (0.05–0.1 mEq / l).
In addition, the interaction of hardness salts with detergents (soap, laundry detergents, shampoos) leads to the formation of “soap slags” in the form of foam. This leads not only to a significant overrun of detergents. After drying, this foam remains in the form of plaque on plumbing, underwear, human skin, hair (an unpleasant feeling of “hard” hair is well known to many). The main negative impact of these slags on humans is that they destroy the natural fatty film, which always covers normal skin and clog its pores. A sign of such a negative impact is the characteristic “creak” of cleanly washed skin or hair. It turns out that the feeling of "soapy" that causes irritation in some people after using soft water is a sign that the protective fatty film on the skin is intact and unharmed. That she slips. Otherwise, you have to spend money on lotions, softening and moisturizing creams and other tricks to restore the skin protection that Mother Nature already provided us with.
However, it is necessary to mention the other side of the coin. Soft water with a hardness of less than 2 mEq / l has a low buffer capacity (alkalinity) and, depending on the pH level and a number of other factors, can have an increased corrosive effect on water pipes. Therefore, in a number of applications (especially in heat engineering), it is sometimes necessary to carry out special water treatment in order to achieve the optimum ratio between the hardness of water and its corrosive activity.
Water hardness is a specific property of water, which is associated with magnesium and calcium compounds dissolved in it, that is, the presence of cations of these elements in water (as the temperature rises, salts of these metals precipitate and form very strong deposits). Water hardness largely determines the suitability of water for use both industrial and domestic purposes. The emergence of scale we are "grateful" for this indicator.
This parameter is calculated as the sum of millimoles of calcium and magnesium ions per 1 liter of water (mmol / l). 1 mmol / l corresponds to the amount of any substance in mg / l, equal to its molecular weight divided by valence. The value of 1 mmol / l indicates the content of 1 liter of water 20.04 mg / l of calcium or 12.1 b mg / l of magnesium. For convenience, use the value of mEq / l, which corresponds to mol / m3. In addition, in foreign countries such stiffness units as German degree (do, dH), French degree (fo), American degree, ppm of calcium carbonate are widely used.
Devices for determining the hardness of water:
There are 3 types of water hardness:
- temporary carbonate hardness, due to the presence along with calcium, magnesium and iron bicarbonate anions;
- constant - non-carbonate hardness, characterized by the presence of sulfate, nitrate and chloride anions, the calcium and magnesium salts of which are perfectly soluble in water;
- total -is defined as the total value of the presence of magnesium and calcium salts in water, that is, the sum of carbonate and non-carbonate hardness.
Water classification by this parameter:
- soft water - 3.0 mg-eq / l and more
- average - from 3.0 to 6.0 mg-eq / l
- hard water - over 6.0 mg-eq / ml.
The reason for the hardness of the water is the underground deposits of limestone, gypsum, dolomite, which dissolve in groundwater, as well as in part, other processes of dissolution and weathering of rocks. Usually in low-mineralized waters, water hardness prevails (up to 70% -80%) due to calcium ions (although in some rare cases magnesium hardness may reach 50-60%). With an increase in the degree of mineralization of water, the content of calcium ions (Ca2 +) decreases rapidly and rarely exceeds 1 g / l. The content of magnesium ions (Mg2 +) in highly mineralized waters can reach several grams, and in salt lakes - tens of grams per liter of water.
In general, the hardness of surface waters is less than the hardness of underground waters. The hardness of surface waters is subject to noticeable seasonal fluctuations, reaching usually the highest value at the end of winter and the lowest during the flood period, when richly diluted with soft rain and melt water. Sea and ocean water have very high hardness (tens and hundreds of mEq / dm3).
Acceptability for drinking depends on local conditions. The taste threshold for calcium ion is in the range of 2-6 mEq / l, depending on the corresponding anion, and the taste threshold for magnesium is significantly lower (in some cases, water with indicators of 10 mEq / l is acceptable). Hard water has a bitter taste and negatively affects the digestive organs, the organoleptic properties of water correspond to a low level.
However, soft water with (less than 2 mEq / l) has a low buffer capacity and, depending on the pH value and other parameters, can influence the corrosivity of the water lines (in this case, increase their stability and performance). In heat engineering, in some cases, special chemical preparation of water is carried out in order to achieve an optimal and effective ratio between the hardness of water and its corrosive activity.
Water hardness is a property of water, which depends on its content of mainly dissolved calcium and magnesium salts. Under natural conditions, these elements get into the water due to the effect of carbon dioxide on carbonate minerals or as a result of biochemical processes occurring in the humidified layers of the soil.
The total content of dissolved calcium and magnesium salts in water is called common stiffness. The overall stiffness is divided into carbonate due to the concentration of calcium and magnesium bicarbonates, and non-carbonate due to the presence of calcium and magnesium salts of strong acids. Since when boiling water, bicarbonates pass into carbonates, which precipitate, carbonate
stiffness is called temporal, or disposable.
Stiffness remaining after boiling is called constant . It is caused by the presence in water of calcium and magnesium salts of other mineral acids (usually sulfates, chlorides, nitrates).
Hardness is expressed by the number of millimole equivalents of soluble calcium and magnesium salts in 1 dm 3 of water (mmol-eq / dm 3). By hardness, water is subdivided as follows: very soft (0 to 1.5 mmol-eq / dm 3), soft (from 1.5 to 4 mmol-eq / dm 3), medium hardness (from 4 to 8 mmol-eq / dm 3), hard (from 8 to 12 mmol-eq / dm 3), very hard (over 12 mmol eq / dm 3).
Carbonate hardness is up to 70-80% of the total hardness.
The hardness of the water is divided into calcium and magnesium. Usually prevails rigidity due to calcium ions (up to 70%), but in some cases magnesium rigidity may prevail.
The hardness of surface waters is subject to noticeable seasonal fluctuations, usually attaining the highest value at the end of winter and the lowest at high water.
Moderately hard water is not dangerous in terms of hygiene, since 20–30% of the calcium necessary for maintaining the body’s normal metabolism enters the body with water. High hardness affects the organoleptic properties of water, giving it a bitter taste and exerting an effect on the digestive organs. Constant consumption of very hard water contributes to the occurrence of urolithiasis. Large amounts of salts in water make water unsuitable for household needs. In hard water, vegetables are poorly boiled soft, soap is consumed when washing clothes, sediment in water pipes falls, etc. Water containing such salts is completely unsuitable for powering steam boilers and for cooling process equipment due to the formation of dense layers of scale on the inner walls.
The permissible total hardness of water for domestic water supply is not more than 7 mmol-eq / dm 3. In some cases, for a particular water supply system, an increase in the total water hardness up to 10 mmol-eq / dm 3 is permissible. For fisheries, total water hardness should not exceed 4 mmol-eq / dm 3.
Separate carbonate and noncarbonate hardness values are obtained using the total hardness and alkalinity values of water. If the alkalinity of water is caused only by the presence of bicarbonate ions in water (the alkalinity of phenolphthalein is zero), the carbonate hardness will be equal to the total alkalinity (see lab. Work number 8). Then non-carbonate stiffness is defined as the difference between total stiffness and carbonate
F necarb. = F total - W carb.
If the alkalinity of water in phenolphthalein is not zero, then only the total hardness is determined.

