Determine the hardness of the water. Water softening
The service life of household appliances depends on the operating conditions. Home appliances working with good quality water will last longer without repair. The use of hard water leads to the formation of scale inside household appliances, which hampers the operation of devices and further leads to their breakdown. Knowing how to determine the hardness of water at home, you can understand how comfortable your household helpers are. Determining the quality of water and taking measures to improve it, you extend the service life of home appliances.
Hard water not only causes scaling. For example, the result of washing in a typewriter also depends on the properties of the water used. On the packages of washing powders even the dosage is indicated, based on the hardness of the water. In most cities, medium hard water enters the houses. This is evidenced by the high concentration of calcium and magnesium ions, which form a layer of scale inside the hot water pipes. 
Water properties also affect the quality of the taste of prepared beverages using home appliances. For example, coffee from the coffee machine or tea from the electric kettle. In soft water, tea leaves brew faster and give off more flavor than in hard water.
Determination of water hardness by external signs
The occurrence of scale on the internal parts and surfaces of household appliances - the most obvious sign of hard water. Late cleaning of scale will lead to breakage of the heating elements in the devices. The presence of limescale on internal surfaces does not allow to accurately determine the degree of stiffness.
Focusing on the external characteristics of water, you can determine its hardness. This is the most simple, superficial method of evaluation. Liquid should:
- be transparent;
- do not contain sediment;
- have no foreign smell and taste.
Water clarity is easily determined at home in this way. Pour into a clean, transparent can of water to a level of 200 mm. Put the jar on the newspaper, if the text can be read by looking through the jar, the water is considered clear.
Know the quality of water will help her smell and taste. The presence of smell of rot in water with a temperature of up to 60 ° C will tell about the content of hydrogen sulfide in it. You can determine the quality of water to taste by boiling it and cooling it to 20 ° C. If there is a sweet taste, then there is gypsum in the composition of the water, bitterness means the presence of magnesium salts, tart indicates the content of iron salts. Smack of rot means the presence in water of decomposed organisms of animal or vegetable origin.
It is possible to check the presence of impurities in this way. On a glass or other hard glossy surface drip with water. Wait until the moisture has completely evaporated and look at the surface where there was a drop. The appearance of a divorce is a sign of water with a high content of impurities. If there are no stains on the glass, then the water is of normal quality. 
To determine the hardness of water on these grounds is very approximate. For a detailed analysis, you can contact the experts who are authorized for such studies. Their analysis will give a complete picture of what kind of water flows from the tap in your house.
Use of special devices
Learn the hardness of water will help a set of special reagents - "Trilon B". It will determine the degree of water hardness with a high level of accuracy. The test kit will additionally detect the presence of other impurities if present.
It is also possible to test the water with special rapid tests, which are sold in pet shops to determine the quality of water in aquariums. Such tests are available and easy to use. The strips from the rapid test kit are sufficient to be lowered into the water for 3-5 seconds and compare the result with the values given in the test instructions. Comparing the values you can determine the quality of water and its level of hardness. 
To determine the hardness of the water will help special analyzers - TDS-meters. Using them at home will help determine the following indicators of water:
- degree of stiffness;
- mineral level;
- amount of salts;
- degree of electrical conductivity.
TDS-meters is convenient to check the quality of the filters and water purifiers used in the household.
Testers TDS-meters are an electronic device that determines the characteristics of liquids. Accurately determine the hardness of the water with the help of TDS-meters does not quite work out. According to the testimony of the device it is possible to indirectly estimate the degree of stiffness. On the basis of the obtained indicators, using the values in the table and formulas that are indicated in the instructions, conclusions are made about the characteristics of the fluid. 
The temperature of the water significantly affects the readings of the TDS-meter, which must be taken into account when determining the stiffness. Frequent use of the device leads to sulfation of the electrodes, which can distort the resulting values of the characteristics of the liquid.
Determination of water hardness with soap
To determine the hardness of water at home is the easiest way to use soap. The method will not give accurate indicators of rigidity, but in general, the result will be enough to make a conclusion about the suitability of water for normal use of household appliances and take measures to normalize it.
You can implement the method using the following steps.
- Crush the soap in the amount of one gram.
- Dissolve the soap in a small amount of hot distilled water. Do not allow the preparation of the solution the appearance of soap suds.
- Pour the solution into a round glass.
- Depending on the percentage of fatty acids in the soap, add distilled water to the glass. If soap is 72%, then add water to a level of seven centimeters, if 60% - six centimeters.
- Pour half a liter of water into a liter jar, the hardness of which is to be determined.
- Pour the soap solution into the jar at the same time.
First, there will be gray flakes on the surface of the water, and then soap bubbles will form. If a stable white foam was formed, then the mineral salts in the water were bound. Control the poured soap solution. Changes in the level of solution remaining in the glass determines the level of water hardness. The less it took to add the solution in a jar of water before the appearance of soap suds, the softer the investigated water. If you pour out all the soap solution from a glass of foam did not form, then the water is very hard.
In the water of increased hardness poorly formed suds. It is caused by the fact that calcium and magnesium ions turn soap into sparingly soluble salts. Poor foam formation from soaps and detergents indicates high water hardness. If the soap is difficult to wash off, then it speaks of too soft water.
Stiffness is one of the most important characteristics of water. This property is given to water by salts of magnesium and calcium (Mg and Ca), which are called hardness salts. The lower their concentration, the softer the water.
Units. There is no single unit of measurement for water hardness in the world. This indicator is measured in degrees, most often used by German, French and American units.
In accordance with GOST 31865-2012, a degree (ºЖ) is adopted in Russia for a measure of hardness, the value of which is equal to 1 mEq / l (numerically, this corresponds to a Ca or Mg concentration equivalent to 1/2 millimole per liter).
To determine the ratio of degrees of different countries use the conventional unit ppm (part per million, or propromille), that is, one millionth of the base mass: 1 ppm = 0.0001% (1 mg per kilogram).
Indications of household appliances are usually expressed in units of the country of origin. In order to compare them with those specified in regulatory documents, the values obtained are transferred to nationally accepted units of measurement.
Values of degrees of rigidity, reduced to ppm:
- 1 dH (German degree) = 17.8 ppm;
- 1 f (French degree) = 10 ppm;
- 1 (English degree, Clark) = 14.3 ppm;
- 1 A (American degree) = 1 ppm;
- 1 mEq / l (1ºЖ) = 50.05 ppm.
Types of water hardness
There are three types of stiffness:
- temporary (carbonate), due to the content of calcium and magnesium bicarbonate - salts of weak carbonic acid. When boiling the salt disintegrate, as a result an insoluble precipitate is formed from calcium carbonate (CaCO3) and magnesium hydroxide (Мg (OH) 2;
- constant (non-carbonate), from which it is impossible to get rid of boiling. In water, salts of divalent metals (calcium, magnesium, barium and strontium), formed by strong acids: hydrochloric (HCl), sulfuric (H2 SO4), nitric (HNO3), are found. Barium and strontium salts for calculating the degree of water hardness are not taken into account, since their amounts are insignificant;
- total is defined as the total concentration of calcium and magnesium ions in water.
In Russia, water is classified according to the total hardness index:
- up to 2 ° W - soft;
- from 2 to 10 ° W - medium hardness;
- more than 10 ° F - hard.
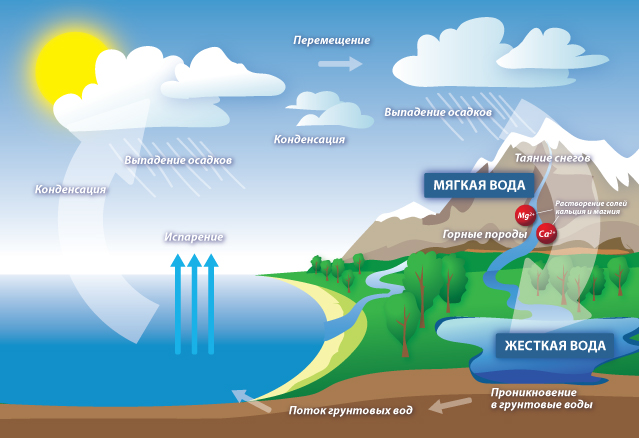 How and why does water hardness change
How and why does water hardness change Why do you need to be able to measure water hardness
Too hard or too soft water can cause irreparable damage to both household appliances and people's health. Soft water flushes calcium out of the body, causing bones and teeth to break down. In such water actively corrode metal surfaces. To avoid this, use a corrosion inhibitor.
Hard water damage:
- creates too much salt load for the genitourinary system. The appearance of urolithiasis, deterioration of the hair and skin;
adversely affects heat engineering and plumbing systems, brings considerable damage to household appliances; - it is necessary to spend more heat on water heating: a layer of scale (sediment from hardness salts) appearing on heating elements has a low thermal conductivity. Due to insufficient heat dissipation, heating elements often burn;
- the consumption of detergents increases due to the fact that the surfactants included in the composition produce insoluble compounds with calcium and magnesium salts and do not form a sufficient amount of foam to remove contaminants;
- the walls of the pipelines are quickly overgrown with lime deposits, therefore the pressure in the plumbing system decreases, the pipes have to be changed.
Watch the hardness of the water coming from the water supply system or local source. If you use the available water softeners, the plumbing system, heaters, washing machines and dishwashers will last much longer.
Before buying expensive devices and reagents, find out which salts and in what quantity are present in tap water. The total hardness index varies with the amount of precipitation, snow melt and other phenomena affecting the salt concentration. To choose the right softeners, first make an analysis to determine the hardness of water.
For domestic purposes: washing, washing and cleaning, it suffices to find out the indicator of water hardness once and, if necessary, use suitable means. For cooking, if tap water is too hard, it is advisable to use bottled water of good quality, which is periodically checked with a portable and easy-to-use device to determine the total and carbonate hardness.
Determination of water hardness by instruments and "by eye"
It can be assumed that water contains a large amount of alkaline earth metal salts by the following features:
- soap and laundry detergent foams poorly;
- limescale is formed on the surface of the heating devices;
- water has a bitter taste and tea is brewed longer than usual;
- when boiling a characteristic film forms on the surface of the water;
- poorly boiled meat products.
1. Strips of hardness of water. Sold in the pharmacy stores "Medtehnika", show the measurement result with an accuracy of 1-2 ° W.
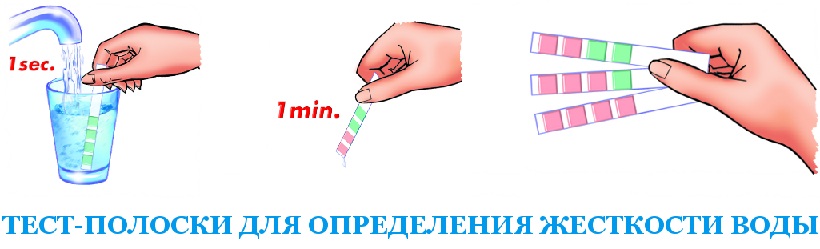
Instructions: lower the stiffness strip into a glass of water, wait until the indicator with which it is soaked change color, then compare with the reference scale.
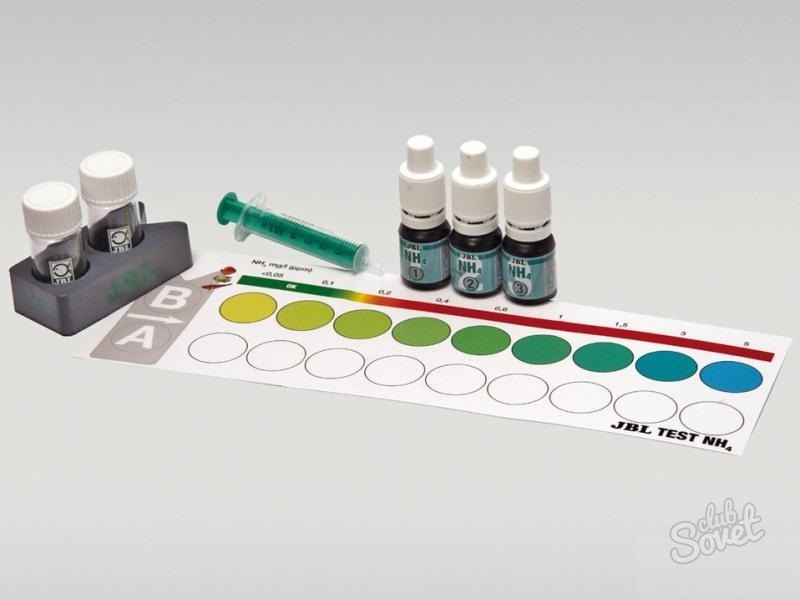
2. Rapid tests for aquariums. This method is based on the titration method.
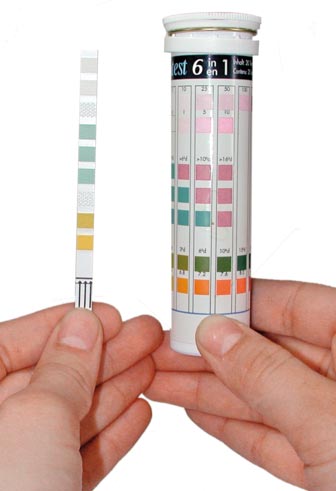 An example of aquarium stiffness test
An example of aquarium stiffness test Instructions: Pour 5 ml of water into the tube and add reagent containing indicator dropwise. The number of droplets of the reagent required for the solution from yellow to turn blue is equal to the number of degrees of hardness, which exactly - as indicated in the test instructions.
3. Special devices. The simplest and most accurate method for determining water hardness is titration. It is based on the reaction of indicators, their ability to change color when a certain concentration is reached in a strictly defined amount of water containing salt. In laboratories, the results of titration are processed using a photocolorimeter.
There are devices whose principle of action is to measure the electrical conductivity of water. The level of conductivity is directly proportional to the concentration of calcium and magnesium salts dissolved in water. On sale there are devices like TDS-meter (total dissolved solids, or "salimeter") and EC-meter (conductometer).
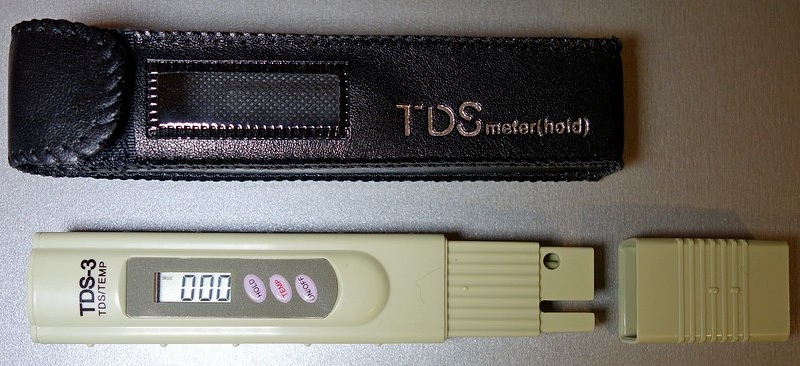 TDS meter example
TDS meter example TDS-meter gives the result in ppm. The device shows the total salt content and the specific conductivity of water. The EC-meter additionally shows the resistivity of the solution in µS / cm (micro Siemens). Result TDS = k * EC, where the coefficient k is within 0.55–0.80 (average value 0.67).
 TDS and EC meter in one device. More expensive, but more convenient
TDS and EC meter in one device. More expensive, but more convenient With the help of such devices it is convenient to monitor and maintain the required water quality in aquariums or for watering plants that are sensitive to increased hardness.
Often there is a need to measure the hardness of water. When connecting a washing machine / dishwasher, gas column and other appliances, the numbers (mmol per dm³) and recommendations for water hardness are indicated on the devices, but for most of these numbers nothing is said. Many do not want to be interested in the hardness of water and very vainly, let us consider why.
What is called water hardness?
Water hardness refers to the salts and trace elements that make up water, some of which can dissolve when heated and boiling, and some do not dissolve or settle. In the water, they fall from the ground. The water from the deep wells is much softer, because under pressure most of the trace elements sink into the surrounding layers of the earth. Why when drilling deep wells in front of a layer of water will necessarily be a layer of limestone or chalk, above and below the underwater lakes.
Increased water hardness has the following disadvantages:
- Enhanced scale formation in heating elements;
- Increased magnesium content gives a bitter taste, vegetables and meat are also badly boiled soft in it;
- Greater energy consumption for heating;
- Worse heat dissipation, soft water in the heating system will heat up faster; the pump will work better since the pipes and boilers will be much cleaner.
How to check the water hardness at home?
The easiest way is to look after the kettle; the thinner the scum, the harder the water. Also, if there is no saturation, and the thirst does not go away after taking the water, it is difficult to wash off non-waterproof cosmetics, this indicates hard water. The heavier the stomach is after water, the harder the water is.
For a more accurate analysis, you can purchase special one-time tests and strips (or Trilon-B, with analogues -Tetra test, etc.), sold in stores with fish and aquarium accessories. For reusable metering there is a more complicated option - to purchase a TDS meter. But it shows not the hardness, but the amount of salts, by which it is possible to determine the hardness indirectly without taking into account magnesium, calcium and other elements.
There is a more practical test that is popular with aquarists - with the help of soap. It is necessary to crumble 1 gram of soap and mix with distilled water in 200 gr. glass to the level of 6-7cm. (if the soap is 60% then 6 cm if 72% -7). Then pour half a liter of test water from the tap into a 1 liter can. Slightly stirring it is necessary to add a solution to the water, and watch when a gray, stable foam appears on top.
If you need 2cm of solution, then the salt level is 4 ° dH. One centimeter of liquid is capable of dissolving 2 ° dH, if after pouring the entire glass of foam did not appear, it means the water is very hard. But to know exactly the hardness of water, including carbonates and all trace elements, at home will not work, for this you need a special analysis and laboratory. But for an approximate analysis of water hardness, it is enough to know the amount of salts.
In everyday life, anyone can face the task of measuring the hardness of water at home. Hardness is the amount of salts of magnesium and calcium, they negatively affect the quality of various processes in which water with increased hardness is involved. In urban water supply, this figure should be about 7 mEq / l, but in fact the norms are often violated. The lower the percentage of salts, the softer and healthier the water. Setting up the dishwasher, the dosage of washing powder, filling the aquarium or a simple interest - that would not serve as a reason for the measurements, this instruction will help you to find out the hardness of the water.
If you wish, you can only compare the quality of water from different sources to choose the best, the easiest way to weigh it: the lighter the water, the softer and more suitable it is for use. You can also brew high quality tea, let it brew, and then evaluate the color and clarity of the drink: the best water in a vessel with a clear peach liquid, and muddy tea indicates contamination. Increased water hardness also affects the quality of washing, washing, because detergents are poorly soluble in such water and therefore less effective. Consequently, another way to check: if the soap foams well, it is even difficult to wash it off - the water is soft.







Do not forget that the quality of the water that you drink depends on your health and the health of your loved ones. Excessive hardness reduces the effectiveness of detergents. Therefore, it is worth being careful and responsible in using water, checking its purity and chemical composition and, if possible, choosing the best one, determining its quality at least at home. In order to reduce the hardness of the water at home, there is a single recipe: you need to boil it before any use.
In order to get a good washing result, you should take into account when dosing washing powder. rigidity (or hardness) of water. There are indirect signs that you can guess that the water in your area is hard - a lot of scale in the kettle and on other electric heaters (boiler, coffee maker and even iron), soap is badly washed, tea is slowly brewing ...
But what if you want more accurate information? Spend a "titration" of your water with soapy water! Such a semi-quantitative method will allow you to estimate the hardness of your water in numbers.
Of course, he does not claim greater accuracy. But on the powder package doses are calculated forintervalsstiffness: less than 7 ° dH (soft), from 7 to 14 ° dH (medium) and more than 14 ° dH (hard). So to determine in what range of values of water hardness is yours, you can.
I found this method on the site of aquarium lovers;"Scientific rationale". It is based on the fact that soap first binds salts of hardness, forming flakes, and only then gives foam. I decided not only to use it to determine the total hardness of tap water in my house, but also to compare its results with the analytical method. My students in laboratory work on ecology and analytical chemistry do this work.


And now the way with soap. For this you will need:
banks - 1 l, 0.5 l and mayonnaise;
ruler;
marker for disks or other writing on glass;
spoon, preferably small, but with a long handle;
If everything is available, then:

 The first bubbles appeared on 3 cm of the solution. And then I expected a “stable foam” like that of a beer, but I barely managed to photograph my resulting foam. It was not truly sustainable even after pouring out the entire soap solution. But still there was foam, and therefore I took 4 cm of a soap solution or 8 ° dH for the result of the titration. The result obtained is close to the result from the laboratory and turned out to be in the same range of rigidity with it. Moreover, the author of the method indicated that the error of this method is 1-2 ° dH and depends on the quality of the soap, distilled water and the skills of the performer.
The first bubbles appeared on 3 cm of the solution. And then I expected a “stable foam” like that of a beer, but I barely managed to photograph my resulting foam. It was not truly sustainable even after pouring out the entire soap solution. But still there was foam, and therefore I took 4 cm of a soap solution or 8 ° dH for the result of the titration. The result obtained is close to the result from the laboratory and turned out to be in the same range of rigidity with it. Moreover, the author of the method indicated that the error of this method is 1-2 ° dH and depends on the quality of the soap, distilled water and the skills of the performer.
In any case, now I know exactly which digits to be used in the label on the packages of laundry detergents.

